Ready-to-use (RTU) containers are gaining momentum in pharmaceutical operations. However, many drug manufacturers are still looking for the optimal method to transfer presterilised containers into the fill-finish area of a filling line.
Machine and technology supplier, Syntegon, and drug containment solutions provider, Stevanato Group, conducted an industry first series of experiments to explore the potential of vaporised hydrogen peroxide (H2O2 or VHP) for outside surface decontamination of RTU vials in trays or tubs/nests. Their results prove that the method increases both flexibility and efficiency during small batch manufacturing.

It comes as no surprise that the market for RTU containers has been growing at a rapid pace during the past few years. Drug manufacturers benefit from reduced time-to-market and total cost of ownership (TCO), as well as increased flexibility and greater integrity of the drug product. From vials to syringes to cartridges, their numbers have increased consistently as more and more pharmaceutical manufacturers turn to small batch manufacturing.
According to a recent market research, the market size for RTU vials alone reached $306.5 million in 2020. With an estimated market growth rate of 14.5% CAGR during the next decade, P&S Intelligence predicts that the RTU vials market revenue will reach $1183.4 million worldwide by 2030.
A growing need for new transfer methods
Although more and more drug producers are turning towards RTU vials, some questions remain unsolved — particularly regarding the appropriate transfer method. In a conventional bulk process, vials are cleaned, sterilised and depyrogenated in a washer/tunnel configuration before the containers enter the fill-finish area. Although it is an optimal fit when producing drugs in bulk, this method reaches its limitations in more than just one way when used during small batch manufacturing.
Although no-touch transfer is practiced globally on several hundred machine applications, experts are constantly searching for new solutions
First, the setup of the machines makes changeovers quite time-consuming. Secondly, energy consumption is high, which can be a major hindrance when frequent batch production is required. Drug producers are therefore looking for a sterile transfer method that is more compatible with small batch manufacturing. Their main objective is to decrease the time needed to perform changeovers, gain more flexibility and thus optimise both efficiency and profitability during production — without affecting safety. Ready-to-use vials offer these advantages.
Improving on no-touch-transfer for RTU vials
Today, the classical way to get RTU vials packed in trays or tubs into the sterile area is the so-called “no-touch transfer.” Before the vials leave the glass suppliers facility, they are packed in a tray or tub/nest, sealed and packed into bags. The tray or tub is protected by one, two or even more layers of (in most cases) Tyvek material, which is required for the final sterilisation process using dry heat, radiation or gas.
All these packaging layers must again be unpacked step by step at the pharmaceutical manufacturer’s filling facility. This is done either in a semiautomated way by an operator within the cleanroom or fully automatically with machines. Each unpacking step increases the risk of contamination. Although no-touch transfer is practiced globally on several hundred machine applications, experts are constantly searching for new solutions. Transferring tools and environmental monitoring material via a fast transfer chamber with a biodecontamination cycle upfront for outer decontamination is common industrial practice. However, the same principle is not used to transfer RTU vials into the isolator … at least not to date.
VHP: tried and tested for decontamination
Vaporized hydrogen peroxide shows great potential. It is already widely used for the inner decontamination of isolators for classical bulk glass vial production on conventional lines. After the decontamination cycle, the isolators in the line are typically aerated with fresh air or by using catalytic converters until the concentration of H2O2 inside them reaches of 1 or 0.5 parts per million (ppm). This is a typical value that makes the isolator “ready for production.”
The vials enter the fill-finish area through the tunnel and are exposed to this minor but still residual concentration in the air. Consequently, gas molecules permeate the containers and accumulate on the inner glass surface of the vial during the fill-finish process. These amounts are, however, low enough to not impact product quality (as in-depth studies have shown).
An industry first series of experiments
The key to understanding whether this method can also be applied to the outer decontamination of presterilised vials is extensive data. Before choosing a new process, pharma manufacturers need to have their most pressing questions answered: do traces of VHP penetrate the packaging during its outer decontamination? Could the H2O2 residue in the vials compromise the quality of the final product? Until recently, there was not enough data available to prove the safety of the VHP method when transferring RTU vials into the fill-finish area with an H2O2 chamber.
In 2021, Syntegon and Stevanato Group joined forces for an industry first series of experiments. By combining the machine manufacturer’s expertise and long-term isolator experience with the drug containment solutions supplier’s in-depth knowledge of RTU containers, the partners examined the effects of H2O2 on RTU vials to better understand the method’s potential for small batch manufacturing.
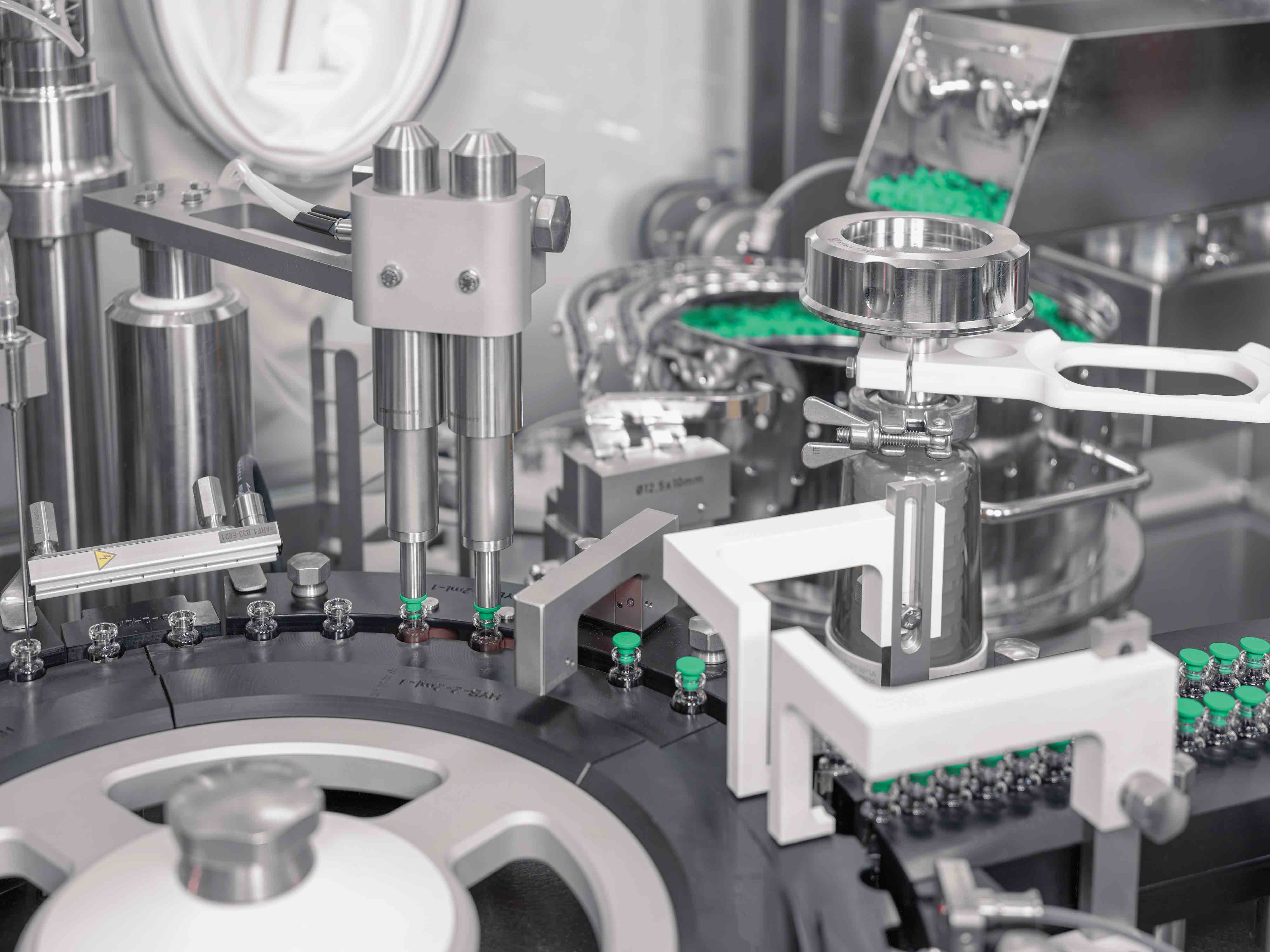
The assumption was that the traces of H2O2 will be absorbed by the outer packaging as well as the presterilised containers. For this method to be safely used with RTU vials, the amounts needed to be low enough to not cause negative oxidation effects on the products inside.
New insights for RTU vials
The experiments took place in Syntegon’s Pharmalab test laboratories in Crailsheim, Germany. The process exposed both plastic and glass vials to a typical H2O2 decontamination cycle in an isolator to test the method and gather reliable and comparable data for both types of the most typical vial materials. For each variant, they used three types of probes: a presterilised, bagged sample vial; a control vial, which had been removed from its outer packaging; and an equally unpacked stoppered control vial. After a short holding time following aeration, the inner vial surface was washed with a control media at different time points to detect the amount of H2O2 in the respective test solutions.
These new insights will lay the groundwork for exciting new possibilities in small batch manufacturing and fill-finish processes for RTU containers
The results of the tests show that multiple parameters, such as packaging, vial size and vial material, duration of exposure as well as aeration time, have an influence on the H2O2 concentration within the container. The data indicates, for example, that glass vials absorbed less vapour than plastic variants. When aerating the isolator until the residual H2O2 concentration inside reaches a standard typical amount of 0.5 ppm, only negligible traces — less than 2 nanomol (nmol) — of H2O2 penetrated the sample vial. These results show that vaporised H2O2 is a safe method for decontaminating the outer packaging of presterilised vials and open the door for a potential shift in the industry.
From theory to practice
In the end, there is no “one-size-fits-all” solution. Each drug reacts differently to the residual concentration in the isolator. Its effect depends on multiple factors, such as the characteristics of the active substance or its formulation. Pharmaceutical manufacturers need to gather precise data first to fully understand these interactions and to evaluate whether vaporised H2O2 decontamination is the optimal method for their production. The experiments from Syntegon and Stevanato Group provide a solid base for data collection, making it easier for drug producers to test their own products and take a data-based decision about their RTU transfer process.
These new insights will lay the groundwork for exciting new possibilities in small batch manufacturing and fill-finish processes for RTU containers.




