Various regulations require cleanrooms to be regularly microbiologically monitored. This applies both to critical production facilities, for example for the aseptic production of medicines, and to less critical ones, such as non-sterile production.
Basically, the more critical the process, the more intensive the monitoring must be. This is usually assessed and defined with the help of a risk assessment and a Contamination Control Strategy (CCS) based on this.
A distinction must be made between surface and air monitoring
In microbiological environmental monitoring, a distinction must be made between surface and air monitoring. The former is usually tested using contact plates or swabs (for more details, see Goverde 2018 or Rieth 2017), while the air is tested using active or passive air sampling. All these methods are established and generally recognised.
Continuous monitoring in the most critical areas (e.g. cleanroom class A and B of aseptic manufacturing or ATMP production) is becoming increasingly important (e.g. EudraLex 2022). So how can it be implemented in the best possible way?
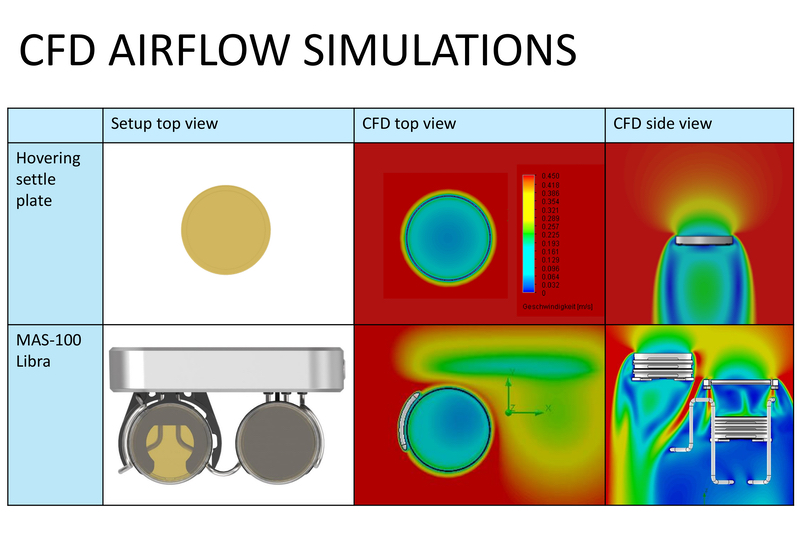
Figure 2: CFD (Computational Fluid Dynamics) airflow study of the MAS-100-Libra. The image shows the airflow or turbulence on the device. In the area of the exposed plate, it is almost the same as in a manually laid settle plate
Simple and cost-effective: passive air measurement with settle plates
The easiest and most cost-effective way to assess the air is with the help of settle plates: Petri dishes with a solid nutrient medium are placed in the cleanroom for a certain period of time, on a surface or in a holder. (For full advantage and disadvantage list see Table 1.)
The air measurement itself is passive: only those particles that "fall" onto the plate by gravity or are blown onto the plate by the air flow are detected. Naturally, these are mainly larger and/or heavier ones. The measurement is therefore rather random, and the accuracy of the quantification is questionable.
Passive air measurement are only those particles that "fall" onto the plate by gravity or are blown onto the plate by the air flow are detected
The exposure time is decisive for the probability of detection of microorganisms. In most cases, the quantity of CFU (colony forming units) per 4 hours is taken as the unit (e.g. EudraLex 2022, FDA 2004).
The results obtained using the settle method tend to be qualitative or semi-quantitative. It is therefore only suitable as a supplement to active measurement methods.
Higher accuracy: active microbial air sampling
In active air measurement, the microorganisms are either accelerated onto an agar (impaction method) or filtered from the air onto a membrane (gelatine membrane filter) or into a liquid (impinger).
These methods quantify the number of organisms per unit of air and can be measured isokinetically. It is therefore considered more accurate than passive air measurement (e.g. Andon 2006 or Goverde 2018).
Fast but costly: alternative air sampling methods / rapid methods
The traditional methods for counting airborne germs are growth-based. It can therefore take between 2 and 10 days of incubation before a result is available.
An immediate result can be obtained with online measuring devices: they detect airborne particles and distinguish microorganisms from inert particles based on their biofluorescence (Biofluorescent Particle Counters, BFPCs).
The exposure time is decisive for the probability of detection of microorganisms
This technology enables rapid reactions to microbiological changes. Nevertheless, it is still rarely used routinely. The main reasons for this are the high validation effort, the difficulties in setting limits and the need to identify isolates in the event of a positive result.
A non-representative survey of pharmaceutical microbiologists therefore also showed that less than half of the respondents would use BFPCs as the preferred method for implementing the requirement for continuous airborne microbial testing.
However, the authorities encourage the implementation of such devices (e.g. EudraLex 2022, Miller 2019, Riley 2004) because they allow continuous monitoring of cleanrooms and faster response to deviations from the normal state.
Continuous air sampling is expected
The requirement of the new Annex 1 (EudraLex 2022) is clear: continuous air measurement must be carried out in critical cleanroom areas.
As described above, this is traditionally done using settle plates, isolated active air measurements with a very low impaction air volume or alternatively with rapid methods.
Various regulations require cleanrooms to be regularly microbiologically monitored
Active continuous air measurement would enable better quantification. However, various regulatory requirements explicitly or at least in principle expect measurement by means of settle plates.
This can lead to the use of settle plates being required during official inspections; this was confirmed to the authors by various colleagues from the pharmaceutical industry.
Table 4 provides an overview of the statements in some important regulatory documents on measurement using settle plates. They show: It is currently almost unavoidable to use settle plates in cleanroom monitoring.

Modern aseptic filling processes are already highly automated. It therefore makes sense to also automate microbiological monitoring, for example with a solution such as RoboCell from Groninger or Versynta from Syntegon. This reduces human intervention and thus lowers the risk of contamination.
The new MAS-100 Libra automated settle plate changer, which can be loaded with up to 6 x 90 mm plates, offers an alternative (Figure 1). This and its unique design offer a whole range of advantages:
- The plates are automatically changed every 4 hours, but other exposure times are also possible. As a result, significantly fewer interventions are necessary during filling, or the filling process does not have to be interrupted at all.
- The minimum coverage time without intervention is 24 hours.
- The MAS-100 Libra can be permanently mounted in the isolator or RABS but can also be used in other zones as a stand-alone device with power supply or battery.
- Compatible with all common agar plates, i.e. the plates used so far can also be used with the MAS-100 Libra.
- A data log file documents all relevant information (e.g. start of measurement, duration of exposure).
- The device takes up little space.
- The MAS-100 Libra automates the conventional settle plate. The measurement therefore remains unchanged and results can be directly compared with historical data. An existing trending can be continued seamlessly.
- The validation effort is very low: in principle, the manual plate is replaced by an automated system. The airflow at the agar plate is not significantly altered using the MAS-100 Libra (see Figure 2).
- The MAS-100 Libra is an innovative solution that combines the classic and proven method of passive air measurement by means of a settle plate with automated monitoring, which is becoming increasingly important.
Conclusion
Classic methods such as passive air sampling with settle plates and active air measurement with a sampling device are available for microbiological environmental monitoring of air in cleanrooms.
Due to the clear requirement of the new Annex 1 (EudraLex 2022) to continuously measure the air in critical areas, both the emerging alternative rapid methods using biofluorescence and the automation of classic methods are gaining in importance.
The MAS-100 Libra presented here is an innovative solution for passive air measurement that combines the traditional settle plate method with modern automation technology.
