Background Information
USP 797 focus on sterile compounding, normally keeping a minimum differential positive pressure of 0.020-inch water column (=5Pa) between each ISO classified area while USP 800 defines the safe handling of hazardous drugs, having a negative pressure between 0.01 and 0.03 inches of water column relative to all adjacent areas.
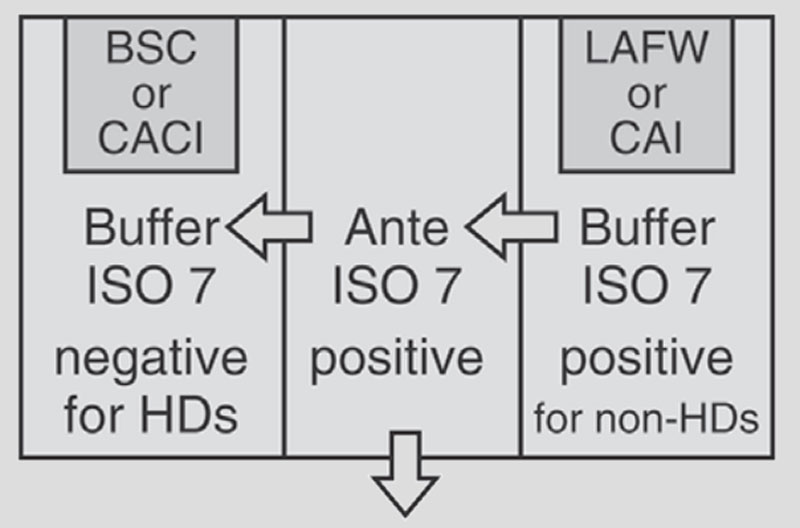
Compounding Cleanroom URS
In order to meet the requirements specified in USP 797 and 800, customer demanded that:
(1) Positive pressure for USP 797 cleanroom;
(2) Negative pressure for USP 800 cleanroom;
(3) Segregated compounding area in USP 800 cleanroom;
(4) Pass through in both USP 800 cleanroom and USP 797 cleanroom
(5) Modular and fabricated structures for cleanrooms
(6) Exhaust system in USP 800 cleanroom and anteroom
(7) Auto sliding doors for buffer room
What you see is what you get
Design to FAT test: Airkey boasts professional cleanroom experts who can customise the cleanroom in consideration of both customer needs and regulations. In addition, the clean rooms are all prefabricated, assembled and tested in Airkey factory to ensure quality and boost confidence.
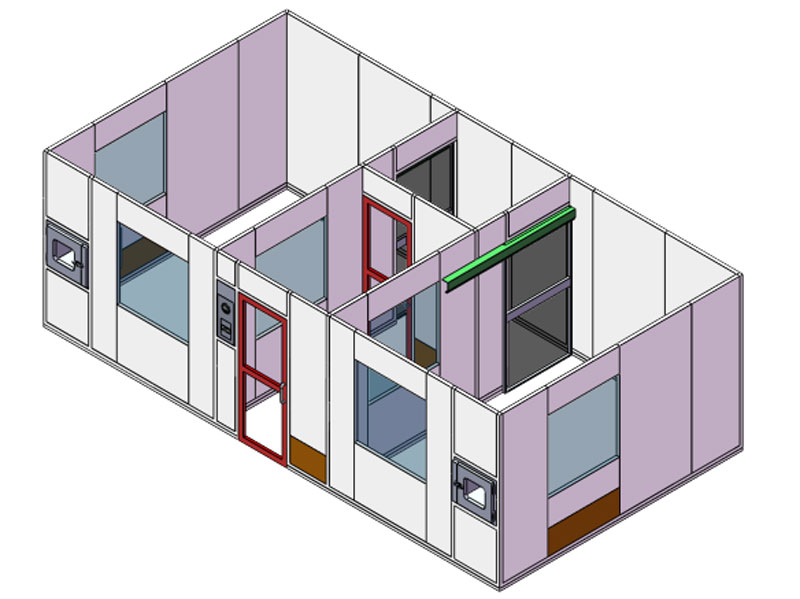
Free-standing structure, fully equipped: the cleanroom is designed and built in a way that it can stand freely without any extra suspension. Proper differential pressures are kept in different clean rooms to protect the operators and drugs. Biosafety cabinets are equipped to act as segregated compounding area for critical handling process. Before any item is introduced into the clean rooms, they will be placed into pass-through. Through double-sided tempered glass windows, the inside compounding process can be overseen and monitored.

Interlocking doors: in the buffer room, 3 interlocking doors are used to facilitate better control of air balance. All doors will never be opened at the same time to avoid cross contamination. In addition, two of the doors are automatic sliding doors as required to realise quicker and hand-free entrance and exit.

Perfection down to every detail: the surface of cleanroom walls, windows, ceilings, doors, frames are all smooth, impervious, free from cracks and crevices and non-shedding so that they can be easily cleaned and disinfected and avoid the accumulation of microorganisms. In addition, aluminium covings are installed between floor to wall and between wall to ceiling.

Results: with all the tests done before delivery and details of photos shared, it is natural Airkey FastIns Modular Cleanroom gets well accepted. This cleanroom will help create a stringent environment for safe and sterile compounding, improving the quality medicines.
The Specifications of this project:
Project: USP797&800 Pharmaceutical laboratory -FastIns Modular Cleanroom
Area: 30 m2
Internal height: 2.5m
Class: ISO 7 (Class 10,000)
Industry: Pharmaceutical
Location: Houston, USA
Website: www.airkeyx.com
Click it for more details about Airkey products.

