Company representative’s involvement went beyond their many discussions with interested visitors at their booth in the VIP3000 area. The company made a high-quality contribution to the LOUNGE’s specialist program with the lecture: “GMP Quo Vadis? Trends in Pharmaceutical Quality Management”.
Experienced industry expert Dr. Michael Beranek was recruited to provide his academic input on the contentious topic of pharmaceutical quality management. This program point was presented as a double feature and highlighted the pharmaceutical QM system, especially with a view to the Consultation Document on Annex 1, Manufacture of Sterile Medicinal Products of the EU GMP Guidelines and its practical implementation.
In addition to a general overview of the EU GMP Annex 1 revision, the lecturer presented the risk-based approach – which occupies a prominent position in the Consultation Document – and substantiated the approach with practical examples.
For application-specific implementation, Beranek highlighted various methods of risk assessment, their possible tools and visualised the practical applications. The advantages and disadvantages of the methods under consideration were assessed along the way, taking into account standardised quality criteria. This gave listeners the unique opportunity to recognise the value of different risk assessment systems for different functions and to see their utility in company-specific applications.
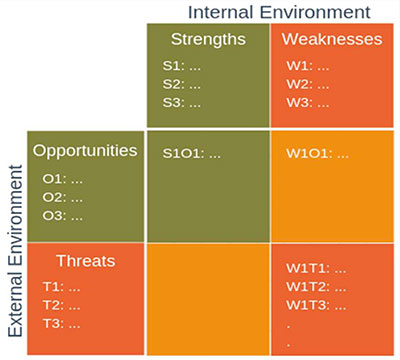
Fig. 1 – Mixed Methods Model
One practical example presented was a microbiological monitoring system design, which took into account the critical influencing factors for the design of the required measuring positions. In doing so, the practical implementation of quality risk methods and their instruments were demonstrated in accordance with the requirements of the EU GMP Annex 1 revision.
For the first time, the practical implementation of the SWOT analysis in systematic relation to a TOWS matrix was shown in detail. It provided a concrete example of a possible risk assessment for pharmaceutical quality management, and highlighted the advantages and disadvantages. This gave the noticeably receptive audience the unique opportunity to experience a linked qualitative and quantitative “Mixed Methods Model” in action (see Fig. 1).
It was shown how the disadvantage of the purely qualitative-oriented SWOT analysis could now be neutralised by linking to the quantitatively oriented TOWS matrix. It offered the possibility of obtaining a key figure-based output from the “Mixed Methods Model” without losing creativity or design freedom during analysis.
With almost 100 attendees, the presentation was one of the best-attended lectures at LOUNGES 2020. The comprei experts present noted the exceptionally positive feedback on the refreshing approach of the double feature, as well as the well-founded input on application-specific implementation. It was followed up by fascinating in-depth discussions that took place at the company’s booth. comprei is looking forward to building on this year’s successful appearance and inspiring visitors in 2021.
