Imagine you are getting ready for your birthday party. You wear your best clothes, visit a hairstylist and be charming as ever! This is what we called “classification” in the cleanroom world: you know that in 10 minutes your particle counter will start and you will have your results for the next six to 12 months. No one wants to fail right? So, clean the “mess”, double check your surface cleanliness, HVAC, return grills and remove unnecessary items.
But, what if someone knocks on your door to deliver a gift early in the morning right after your very late birthday celebration night? Even while you are still sleeping? Can you imagine! You are in pyjamas with messy hair! This is what we called “monitoring”: you fully focus on your production routine, but there are also several contributors to your production quality, especially viable and non-viable contamination aspects. Under these circumstances, you should have your particle levels within the acceptable level without knowing what is waiting for you in your production routine.
Based on this analogy, you cannot wait until the early morning with your best clothes and hair for a potential visitor, but at least you can be ready for any of your friends to visit early morning. In the sterile manufacturing world, we should be prepared for any unexpected situation, and that’s why we have monitoring systems. They are like our camera and infrared sensor reminding us as early as possible to get ready for these “unexpected guests”.
Let’s start with classification (the birthday party) to get a better understanding.
Cleanroom classification
As mentioned in various standards and regulations, such as EU GMP Annex 1 and the US FDA Aseptic Processing Guideline, cleanroom classification as per ISO 14644-1 is essential for continuous compliance. Cleanrooms and clean air devices should be classified following ISO 14644-1. Classification should be clearly differentiated from operational process environmental monitoring. Portable particle counters are used for cleanroom classification.
Now, consider the following example of classification calculation: A cleanroom has a floor area of 30sqm and is specified to be ISO Class 5 in operation. The classification is to be performed using a discrete particle counter having a flow rate of 28.3 litre per minute. One particle size will be considered: D1 ≥ 0.5 μm
Now, define parameters from table, equation and known data. First, write down all known data:
- Room Area ► 30sqm
- Targeted Class (N)► 5
- Particle counter flow rate ► 28.3 LPM (1 CFM)
- Particle Sizes Considered ► D1=0.5 μm
Now, let’s get data from tables;
NL = 7 locations, (As per Table A.1)
Cn,m (≥ 0,5 μm) = 3520 particles/m3 (As per Table 1)
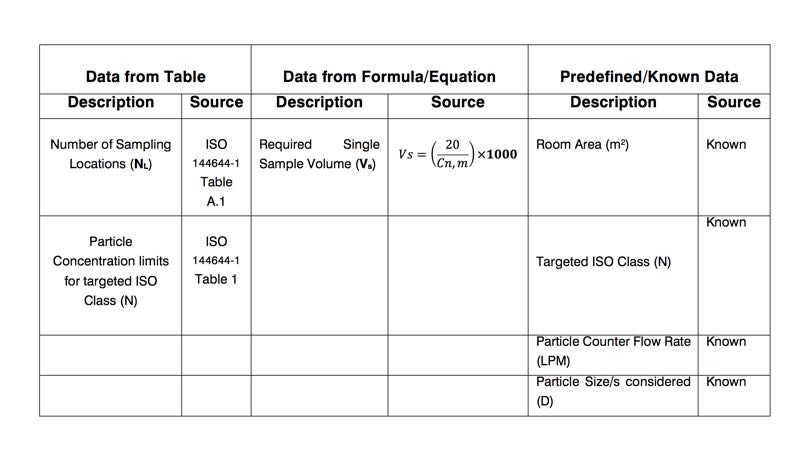
Table 1
Second, calculate minimum single simple volume, Vs ;
Vs=(20/(Cn,m))×1000
Cn,m (≥ 0,5 μm) ► Vs=(20/3520)×1000=5.68 litres
The single sample volume has been calculated to be 5.68 litres. As the light scattered airborne particle counter (LSAPC) being used for this test had a flow rate of 28.3 litres per minute, a minimum 1-minute single sample count would be required, and therefore 28.3 litres would be sampled for each single sample volume.
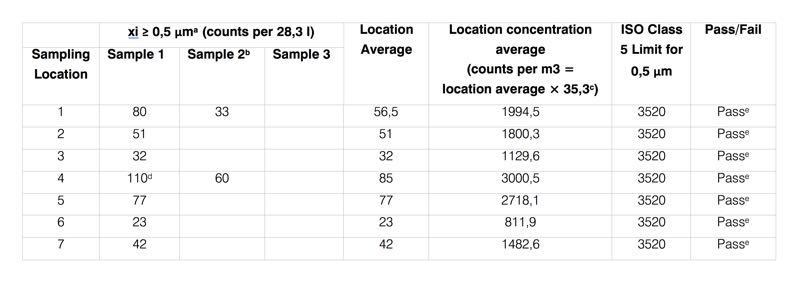
Reporting: Prepare sampling data table for 0.5 μm particle size
For this example, single particle size 0.5 μm is considered. However, more than one particle size suitable for targeted ISO Class mentioned in Table 1 (ISO 14644-1:2015) can be selected.
In this case, the same sampling data table should be prepared. Each line in all tables should pass to achieve targeted ISO Class.
For the same sampling location, an additional sample can be measured, and the average can be calculated.
In many cases, undesired interventions such as isoprobe/tubing contact, at rest condition interruptions, or as per sampling plan etc., can lead to multiple single sample volume.
If multiple single sample volumes are taken at a sampling location, the concentrations are averaged and the average concentration must not exceed the concentration limits given for the specified ISO Class targeted.
Here, 35.3 is the multiplier to reach 1000 litres as per particle counter flow rate, which is 28.3 LPM (35.3 x 28.3 = 1000 litres). For 50 LPM particle counters, this multiplier will be 20 and for 100 LPM devices, 10.
At location 4, one of the individual particle count concentrations does not meet the limit established in Table 1; however, the average particle concentration for location 4 does meet the limit.
The average particle concentration for each of the sampling locations does meet the limit established by application of calculation table. Therefore, the air cleanliness by particle concentration of the cleanroom meets the required ISO Class.
Cleanroom monitoring
Non-viable airborne particle monitoring is essential. Particles are significant because they can enter a product as an extraneous contaminant and can also contaminate it biologically by acting as a vehicle for microorganisms.
There are different particle monitoring systems with remote locations such as manifold and online monitoring systems.
For manifold systems, the particle counter should be connected to a manifold unit, which changes sampling locations per defined time, such as per minute. Between each sample, there is a buffer time that allows the particle counter and manifold to clean the sampling pathway.
Understanding online monitoring
These consequential sampling manifold systems are not suitable for monitoring a sterile pharmaceutical cleanroom because monitoring should cover all durations for each sample locations without delay or interruption.
But, different than manifold systems, online particle monitoring systems have independent particle counters, with a sampling isoprobe in each critical location. Without delay and/or interventions, particle monitoring can be undertaken for the full duration of critical processing, including equipment assembly in each selected monitoring location.
When we say online monitoring, most of the people consider it to be a 7 day/24 hour approach, which is wrong. Online monitoring means before, during and after production with a reasonable time frame to cover preparation, filling/production and the cleaning cycle.
Location, location, location
For cleanroom classification, the minimum number of single sample locations are defined based on EU GMP Table A.1. The cleanroom should be divided into zones of equal size, and sampling locations should represent characteristics of each zone. For cleanroom monitoring, sample locations should be defined based on formal risk assessment.
Each representative location should be set and verified based on historical data, trend and production routines.
Normally, this representative location is not more than 30 cm away from the work site, within the airflow and during operations.
The FDA's aseptic processing guideline recommends that measurements to confirm air cleanliness in critical areas be taken at sites where there is the most potential risk to the exposed sterilised product, containers and closures.
The particle counting probe should be placed in an orientation demonstrated to obtain a meaningful sample. Regular monitoring should be performed during each production shift.
Non-viable particle monitoring should be conducted with a remote counting system. These systems can collect more comprehensive data and are generally less invasive than portable particle counters available today.
How to select locations for monitoring
The main considerations to select locations to be sampled are:
- All locations where there is a possibility of operator intervention, for example access points to the Grade A environment
- Original room classification studies, qualification studies and the rationales for previously used sampling/monitoring arrangements
- Areas where there are normally no interventions, but sterile components/products are still potentially exposed to airborne particulate contamination due to abnormal interventions or for other reasons
- The length of time that sterile components and/or products are exposed during processing: an example might be stoppers in a feed hopper. In this instance, there is little risk of intervention. However, the stoppers may well be sitting exposed in the hopper for some time, so that there is a potential for build-up of particulate contamination over time. It would, therefore, be good practice to sample air at this location to demonstrate continued compliance with the air quality being delivered to the components over the processing time.
In this case, critical sample areas to be considered are:
- The point of fill
- Component hoppers
- Inspection hatches
- Descrambler tables
- Stopper and capping stations
- Loading of freeze driers
- Unloading of sterile components which are not protected by autoclave bags
- Interfaces between equipment and the Grade A area
- Isolator transfer devices
- Aseptic manipulations
- Operator interventions
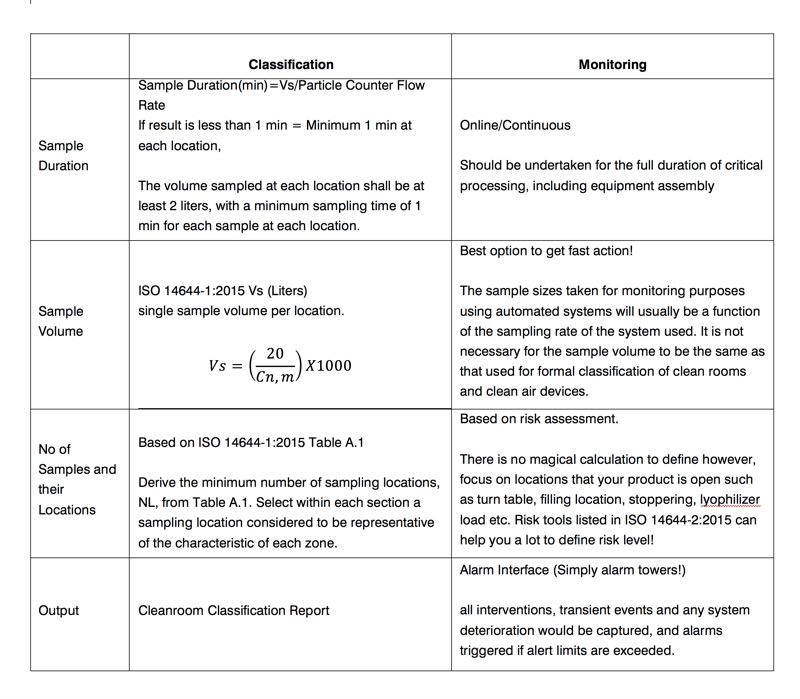
Table 3
Table 3 highlights the main differences between classification and monitoring based on sample duration, sample volume, and the number and output of sample locations.
This article is featured in the November issue of Cleanroom Technology.

