A critical cornerstone of quality control (QC) in drug manufacturing is the monitoring and detection of microbial contamination in the environment and in samples taken throughout the process. Rapid turnaround times and high sensitivity are essential for microbial QC testing as a contamination event can delay or shutdown a production line for a significant amount of time to allow for a proper investigation and corrective action.
Unfortunately, traditional microbial QC tests can require more than a dozen steps and five to seven days before results are available (figure 1A). In the traditional approach to QC, a sample is collected and then poured into a funnel; vacuum pressure is applied to pull the sample through to a membrane which is placed on agar, sealed with a lid and placed in an incubator. With environmental monitoring, the agar is in the form of a contact plate which is touched to a surface, sealed and incubated. Up to seven days may be required for visible evidence of the presence of organisms.
Automated approaches can support digitisation initiatives
Following incubation, plates are read manually, which can be quite tedious for a technician when processing perhaps 500 to 1,000 plates per day. Intense focus is required and a very small colony on just a handful of plates can be easily missed. This process has been made even more labour-intensive as the FDA requires that one technician reads a plate and a second verifies the count, adding to the time and cost of processing samples.
By the time a traditional microbial test is completed, it can be difficult to rewind several days into the past and pinpoint the cause of a contamination event. Given the cost of goods used in the biopharmaceutical manufacturing process, any delay in identifying a contamination event has a significant financial impact.
Innovative approaches to microbial QC that are faster and deliver the accuracy needed to support confident decision-making are enabling adoption of next generation manufacturing strategies while meeting regulatory requirements and helping to ensure product quality and patient safety. These technologies for detection of microbial contamination are addressing the shortcomings of traditional processes which are too time- and labour-intensive to keep pace with the modernisation and acceleration of the bioprocess workflow.
Rapid methods
New technologies are available to accelerate the process of QC microbiology and reduce the time required to identify a contamination event. These innovations include the ability to detect colonies on agar plates before they can be seen by a technician, enabling a significant reduction in the time required to identify a contamination event (figure 1B).
With traditional methods, the colony must be large enough to be seen by the human eye, which can be on the order of 10 million cells per colony. Most rapid technologies use methods which incorporate a chemical reaction, treat the sample in some manner, or lyse the cell to release cellular contents such as ATP - in all cases, these techniques are used to amplify a signal that permits earlier detection. Unfortunately, these methods either destroy the cells in question, eliminating colony traceability, or require extensive sample preparation, trading one laborious task for another.
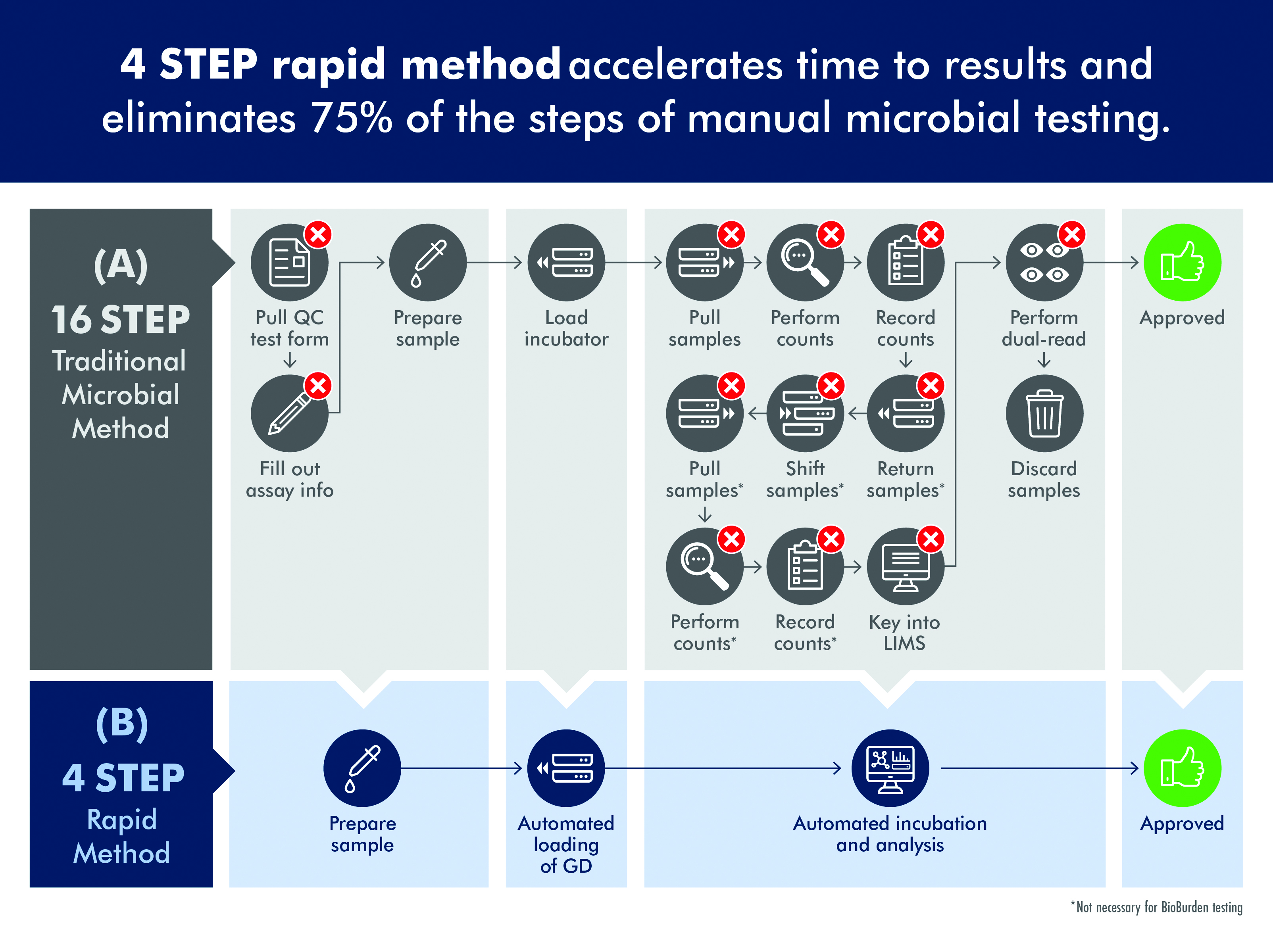
Figure 1: Traditional microbial methods are time consuming and labor intensive while rapid, automated methods significantly reduce the time to result
A more direct approach leverages detection of molecules within bacteria, yeast and mould that fluoresce under blue light. With this approach, only 8 yeast cells or 50 - 100 bacteria are needed to generate a signal, in contrast to the 10 million needed to make a colony visible to the naked eye. An important advantage of this approach is that it is non-destructive and allows tracking of the cells over time to confirm whether they are growing into a viable colony.
Figure 2 summarises these and other rapid methods and provides a comparison of the key benefits that each approach delivers as bioprocessing facilities are modernised, while remaining compliant with regulatory requirements.
Batches can be prevented from being sent down a contaminated production line
Automation of rapid microbial testing can provide further advantages in speed, capacity and throughput. Importantly, automated testing helps to ensure data integrity, which encompasses the accuracy, consistency and security of QC test results. Automated approaches also enable more complete integration into manufacturing processes. QC results can move directly into the LIMS system, enabling greater control, effective data tracking and supporting digitisation initiatives.
Considerations
There are several options for rapid QC microbiology and each can be leveraged for different combinations of environmental monitoring, bioburden testing and sterility testing as shown in figure 2. When evaluating options, an assessment of the type of testing that will be needed now, and in the future, should be conducted. Use of the same rapid format for all environmental monitoring, bioburden testing and sterility testing within the manufacturing facility can provide a great deal of flexibility and reduce the amount of training required for QC team members. Importantly, the testing methodology should also be evaluated in terms of whether it has a robust history of regulatory acceptance and a track record of success within the biopharmaceutical industry.
A critical success factor when implementing an automated rapid method is validation of the system in terms of accuracy, precision and equivalence with traditional methods. While the process is relatively straightforward, successful validation requires planning, resources, and management, all of which should be offered by the technology provider. A skilled validation team will provide a complete suite of validation documents, services, and project management to ensure timely qualification of the system, encompassing all the steps in the validation process:
- Installation and operational qualification (IQ/OQ)
- 21 CFR Part 11 assessment
- Performance qualification
- Time-to-result analysis
- Method suitability
Growth-based systems can be validated using an automated compendial approach as specified in PDA Technical Report 33 (TR33) and USP Ch <1223>, and with robust project management services, validation can be completed in less than six months.
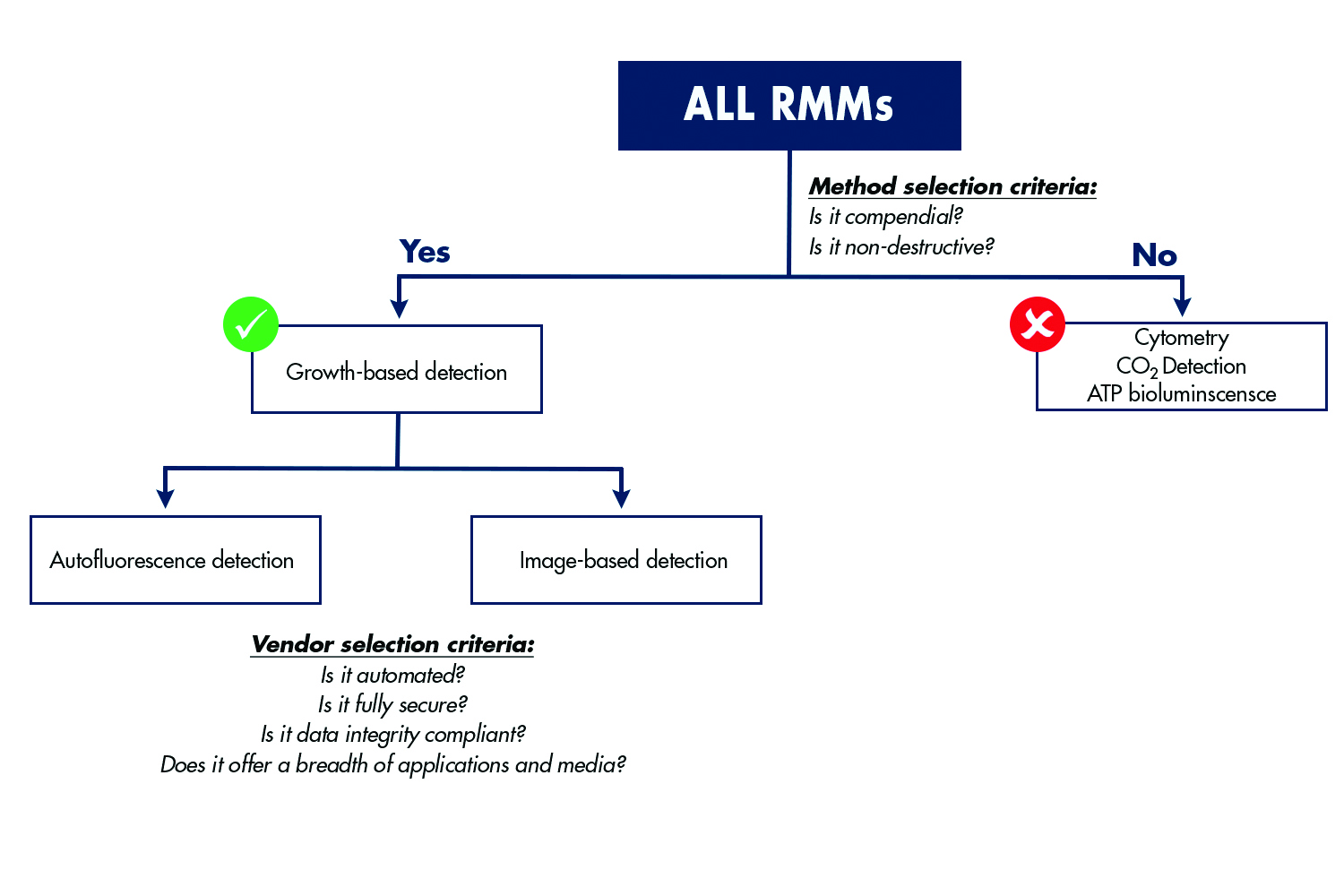
Figure 2: Several rapid technologies are used for QC microbiology. It is critical for QC departments to evaluate several key criteria as they plan for modernisation of manufacturing
A key step towards modernised manufacturing
Multiple trends in the biopharmaceutical industry - including significant growth in complex biologics manufacturing, emergence of continuous processing and modular manufacturing and acute demand for products to address the ongoing coronavirus pandemic - are driving the need for modernised pharmaceutical manufacturing. To support confident decision-making and keep up with these general trends, QC processes must become faster, implement automation, and ensure complete data integrity.
Rapid compendial technologies have received approval from either the FDA or EMA for use in cGMP environments
Rapid methods for QC microbiology enable a faster response time in the event of a contamination. Batches can be prevented from being sent down a contaminated production line, the actions of a technician performing a task incorrectly can be addressed and equipment can be flagged for cleaning more quickly. For manufacturing sites that have multiple product lines, changeovers no longer must wait several days for the results of QC microbiology tests. If that transfer process is shortened by just one day, it can translate into an extra batch of product produced over the course of a year.
Given the push to modernise and make bioprocessing workflows more efficient, the time is right for companies to consider adoption of rapid QC microbiology solutions. Rapid compendial technologies have received approval from either the FDA or EMA for use in a cGMP environment. When combined with advanced automation, this strategy enables fast, accurate identification of contamination events, eliminates manual sample analysis, boosts QC lab productivity and enhances data integrity.
N.B. This article is featured in the September 2020 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.




