Microbial enumeration testing in the pharmaceutical QC lab is based around well-proven traditional manual methods that have changed little since the creation of the first pharmacopoeia. These manual processes rely heavily on the training, expertise and judgment of the individuals who perform them.
Improvements have been made to the processes to minimise variation such as: use of commercial media, validated incubators, verification signatures and electronic batch records; however, at the core, the ability to accurately see and enumerate colonies then accurately record the information to paper or electronic system is still a weak link in the forensic data trail.
It remains one of the few QC processes where sample collection, processing and result collection followed by disposal of the final raw data can be performed without supervision or traceability.
Added to this are the pressures on staff to turn around results quickly that are in compliance. All factors add to create two high-risk scenarios for the integrity of records.
Time stamps for each count permit reviewers to determine if sufficient time was permitted for an accurate colony count
First, the preparation and transport of samples, and second, the incubation, enumeration/data transfer stage. The latter portion is concerned with the integrity of the data for discussion in this paper and the "ease to audit the process" from a reviewer/auditor viewpoint.
In their 2017 paper, Platco and Cundell discuss a number of data integrity issues that might occur within the microbiological laboratory, noting the historically manual operations and their attendant risks of recording and arithmetic errors, including the difficulty in detecting these errors due to data review after the events occur. Until recently, microbiological methods have seldom been implemented using automated technologies.
A regulatory game changer
Although 21CFR211.194(a)(8) requires original records to be reviewed for "accuracy, completeness, and compliance with established standards", deficiencies in the current (historic) state of manual microbiological methods have been exposed with the enhanced focus on data integrity by regulators in the past decade. In two papers, De La Torre (2019) and Unger (2020) both note the increase in data integrity-related observations, especially since 2015. Between 2015-2018, data integrity issues were present in 70-90% of FDA Warning Letters.
Data Integrity focused guidance documents from MHRA and FDA among other regulatory bodies, emphasise the importance of understanding process risks and mitigating them to acceptable levels. Manual processes are difficult to mitigate, because they require people to follow them and leave little evidence of non-compliance to be discovered after the fact.
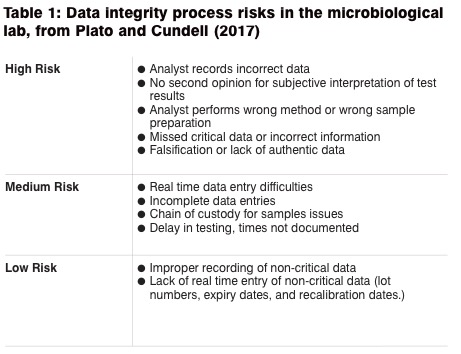
Platco and Cundell provide a list of risks in the microbiological lab, applicable to most compendial tests performed. Table 1 looks at these data integrity process risks in the microbiological lab. In their opinion, the microbial tests at greatest risk for data integrity issues are: "sterility test, the gel clot Limulus Amebocyte Lysate (LAL) bacterial endotoxin assay and any microbial enumeration test which involves counting microbial colonies which covers most of the other compendial tests."
PDA Technical Report 80 provides a number of data integrity observations including sampling, sample containers and pre-test storage, excessive use of disinfectants, inaccurate sampling due to inadequate equipment maintenance and calibration. They recommend that plates be retained after counting to permit second person verification to rule out data integrity issues.
Regulators will continue to examine laboratory operations and data closely, to assure that reported test values are accurate, complete and trustworthy. Assuring that processes are followed requires an additional level of review than has previously been applied to manual processes. This additional review adds additional demands on senior microbiologists and adds time to the release process.
The current state: microbial plate counts
In addition to the data integrity risks of manual techniques, hours at a laboratory bench can create user fatigue resulting in reduced accuracy, requiring analyst breaks. Fatigue can be reduced by adding additional analysts, but this requires significant time investment in training and on-going effort by all analysts to be consistent.
In his 2020 review of microbial colony counting, Tim Sandle reported the result of a study where two analysts differed in counts by as much as 50% when counting beads in agar to represent colonies. Additionally, he describes other factors that influence an analyst's ability to effectively count plates, including plate position, type and direction of lighting.
In addition to the challenges above, plate count results can only be retained and second person reviewed for few hours, after which total counts may change due to the appearance of slow-growing organisms, spreading organisms or contamination from the plate reading process. In the past, it was common for lab personnel to review the documentation of plate counts and not the plates themselves, choosing to accept the risk of inaccurate counts. Due to data integrity concerns and regulatory citations of inaccurate plate counts, labs must now implement second person review of all plates or find alternate technologies to conduct the methods.
In addition to time limitations for original sample retention, contemporaneous time stamps for start/end of each count permit reviewers to determine if sufficient time was permitted for an accurate colony count but are seldom recorded in manual counts.
Manual processes are difficult to mitigate, because they require people to follow them
For example, an FDA Warning Letter to Hospira Healthcare India in March 2019, stated: "Your firm also stated that, after implementing extra plate- reading oversight in the microbiology laboratory, a notable increase in counts emerged in environmental monitoring results, with a particularly "sharp" increase in personnel monitoring excursions across the facility."
Today's automated plate counting technologies, in contrast, offer unprecedented capabilities to improve consistency, save time, reduce analyst fatigue, capture data automatically and fully intact, and control reviewer access with unerring documentation - in short, offering myriad ways to satisfy the stringent data integrity standards now driving the pharmaceutical industry.
In a continuation of this paper next month, we will outline the comparative advantages to be realised by QC labs that evolve from manual plate counts to automation, while suggesting key considerations for optimal implementation.
Moving forward: digitalisation
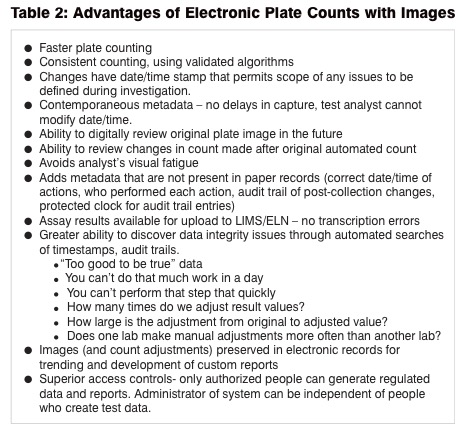
The key to mitigating microbiological risks, increasing efficiency and reducing ergonomic issues can be found with the use of digital systems. Digital systems offer hands-free, consistent analysis of plates, the capability to save an image of the plate at the time of analysis, and the ability to save a complete record of testing whether passing, failing, or invalid. Table 2 lists some of the advantages of digital plate reading and analysis
To read more on the key considerations and best practices for digitising microbial enumeration, make sure to check out the follow-up article in Cleanroom Technology's April issue!
