Food processing is a hygiene-critical activity; precautions are necessary to minimise microbial survival and growth. To achieve clean surfaces and environments during food processing, a systems-based approach is essential. This includes effective cleaning and sanitisation, workflow optimisation, environmental controls, and the integration of antimicrobial technologies where appropriate.
Key definitions
“Visual clean” refers to a surface being clean to ‘‘sight and touch’’. The presence of organic matter on food contact surfaces (FCS) can support microbial attachment and create a food safety risk. Proper surface cleaning and sanitising are essential safeguards against foodborne illness. While visual clean is a basic requirement in the food processing environment, microbiological clean is only attainable with sanitising after a thorough cleaning to remove food residues and other organic materials.
Most cleaning products contain non-ionic surfactants (superior emulsifiers and detergents for oily soil), anionic surfactants to lift and suspend soils, or a mixture of both in their composition
“Microbiological clean” is the absence of bioindicators of contamination risk by foodborne pathogens. While most local health departments utilise visual, but not microbiological methods when inspecting food processing operations, periodic microbiological evaluation of high-risk food service operations is recommended to support visual inspection and minimise the risk of foodborne disease outbreaks.
“Cleaning” is the initial critical step to physically remove visible debris, dirt, and dust. However, cleaning agents vary in their ability to remove different soil types. Thus, the correct choice of cleaning agent is essential to effectively clean a food processing facility.
Most cleaning products contain non-ionic surfactants (superior emulsifiers and detergents for oily soil), anionic surfactants to lift and suspend soils, or a mixture of both in their composition.
Cleaning alone contributes favourably to the cleanliness of the food processing environment because allergens and microorganisms are being removed from surfaces. On the other hand, a sanitising agent on an FCS has an efficacy rate of 99.999% in 30 seconds, and 99.9% efficacy rate in 30 seconds on a non-FCS.
While the use of sanitisers is better than cleaning alone, the reduction of pathogen populations on surfaces is exponentially better when disinfectants are used. Disinfecting a surface destroys or inactivates microscopic organisms as claimed on the label of a product.
The minimum level of effectiveness in a modern day disinfectant is 100% kill of 10-6 initial population of an organism. Table 1 reveals the efficacy of decontaminators is dependent on target microbial species, pH, presence of biofilms, temperature, concentration, and contact time.
Food manufacturers are encouraged to incorporate environmental monitoring programmes to confirm the efficacy of their cleaning and disinfection process. The labels on cleaners, sanitisers and disinfectants include directions for product preparation and use.
These instructions are derived from careful testing and determinations of efficacy and developed through a regulatory process. Therefore, failure to follow instructions could result in diminished product performance, maintenance of microbial populations on surfaces, and unintentionally spreading microbes.
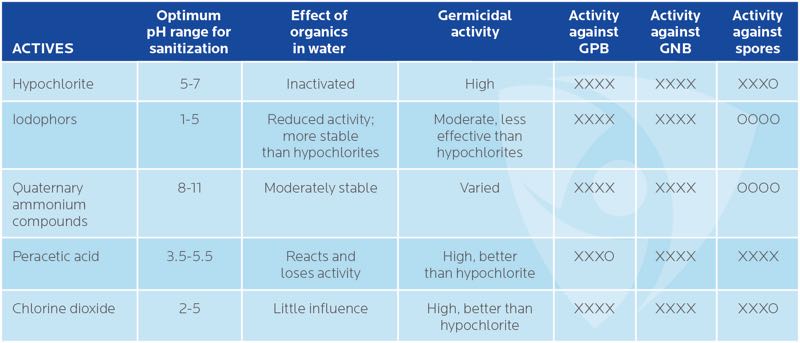
Table 1. Efficacy of common actives used in sanitisers and disinfectants
XXXX – Highly effective | XXXO – Moderately effective | OOOO – Ineffective
Management and optimisation
The food industry application of good manufacturing practices (GMP) requires a holistic approach from the entry of raw materials to the handling of finished products. Proper design of the food processing facility is critical for the reduction of environmental contamination and microbial load. While facilities for each food type will have special design requirements, some general guidelines will apply to all types of food processing plants.
Poor design and location of HVAC systems introduce problems into the food processing environment if air flows from contaminated to clean areas
The main guidelines to prevent entry and transmission of pathogens into the food processing environment are to isolate the raw materials, receiving area and associated personnel from the processing and packaging areas, and ensure there are no direct access points.
Figure 1 represents the recommended design of a food manufacturing facility to control cross-contamination hazards. Facility design is then supported by smart process flow, daily cleaning and sanitisation, and effective pest and insect control.
Food processors must also consider structural factors that can contribute to contamination events. The food processing environment should employ the correct use of natural or mechanical ventilation to minimise airborne contamination of food, control ambient temperatures, odours, and humidity.
Heating, ventilation, and air conditioning (HVAC) systems provide indoor environmental comfort based on principles of thermodynamics, fluid mechanics and heat transfer. However, poor design and location of HVAC systems introduce problems into the food processing environment if air flows from contaminated to clean areas.
The placement of hygiene infrastructure is also essential to safeguard the food processing environment. Foot baths and hand sanitising stations are valuable structures to support decontamination before entering hygiene-critical areas. Accessible restroom facilities are needed for the staff, however, their proximity and site relative to food processing areas should be carefully considered.
Antimicrobial protection
There are some circumstances where it is challenging to maintain microbiological clean critical surfaces because they are continuously exposed to contaminating elements, or there is limited physical access due to their structure, location, and/or material construct.
Critical surfaces that are potential candidates for incorporation of antimicrobial technologies include shelves, tables, bins, conveying elements, walls, isolation curtains, and air filters. Antimicrobial incorporation offers an additional level of protection for hygiene-sensitive systems.
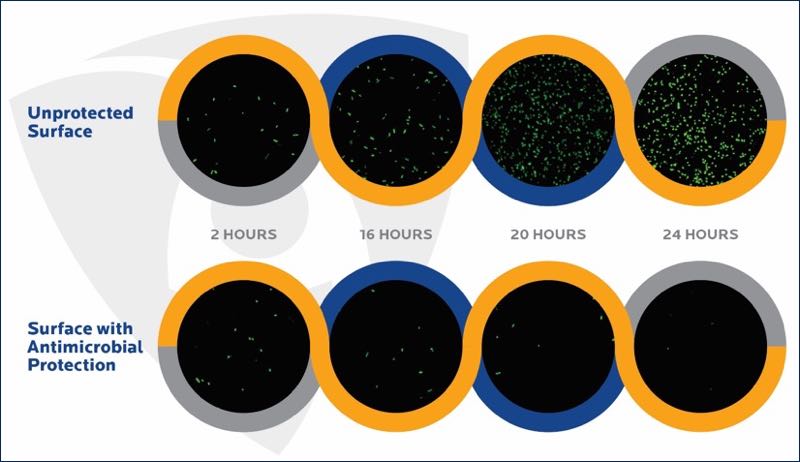
Figure 2. Survival of bacteria on unprotected and antimicrobial-protected surfaces (internal Microban study, confocal fluorescence microscopy)
Many factors influence the choice of a suitable antimicrobial. The food processor must ascertain the surfaces that will benefit from incorporated antimicrobials and evaluate the microbiological risks associated with surfaces and materials. For example, food conveyor surfaces will benefit from antibacterial treatment, while air filters and construction materials such as caulks and sealants will require antifungal performance.
For durability and stability, antimicrobials are incorporated into products during the point of manufacture or as a post-manufacture coating.
Regulatory compliance
Regulatory considerations are also important because allowable claims are location-specific. In the European Union, incorporation of antimicrobials into food contact and non-food contact surfaces is regulated by the EU Biocidal Products Regulations’ (BPR) Regulation (EU) 528/20126. Antimicrobials, including those for food contact considerations, are appropriately classified and migration limits declared.
In the United States, antimicrobials are registered with the Environmental Protection Agency (EPA) under the Federal Insecticide, Rodenticide and Pesticide Act (FIFRA). It is important to obtain reliable guidance about regulations that monitor antimicrobial technology supplier claims and enforcement. Table 2 shows examples of products containing antimicrobial technologies.
The value of incorporated antimicrobials is that they support microbiological clean surfaces between visual cleanings and protect foods from microbial contaminants that could cause food spoilage and/or risk foodborne disease outbreaks.
Some bacteria can replicate rapidly and when unhindered they can double their population in 20 minutes.
Surfaces with built-in antimicrobial protection are continuously challenging the ability of microbes to survive on surfaces, therefore these surfaces are microbiologically cleaner than unprotected surfaces.
Disclaimer: Products featuring Microban technology are sold worldwide. The data provided in this article serves exclusively as a source of general information. Due to regulatory differences, not all of the claims information provided in our materials is valid in all countries or regions. Products featuring Microban technology may only be marketed, sold and used in conformity with official regulations, including restrictions and advertising claims, pertaining to the specific country/region where the product is being marketed, sold and used.
This article is featured in the November 2018 issue of Cleanroom Technology. The digital edition is available online.
