Oxygen depletion can be used in rapid microbiology assays to test surface microbial contamination in cleanroom environments and is an integrated component of certain rapid microbiological methods (RMMs).
Microbial contamination can be detected based on measurements of oxygen depletion over incubation time in a pharmacopoeia-recommended liquid broth, such as tryptic soy broth (TSB).
Potential applications for this technology include testing microbial bioburden in raw materials, excipients, drug products and pharmaceutical water.
This technology can also be applied to environmental monitoring applications in the pharmaceutical industry.
Results from the use of this technology demonstrate that this particular RMM is reliable, sensitive and equivalent to standard compendia tests.
Monitoring pharma environments
Regulatory requirements for monitoring microbial contamination in pharmaceutical manufacturing environments include: a detection system for total air particulates and microbes (viables); a monitoring plan providing information about the facility’s state of control; short and long-term data evaluation; control of heating, ventilation and air conditioning (HVAC), high-efficiency particulate air (HEPA) filters, differential pressure issues; and information on the amount and types of microorganisms recovered in the facility.
Both the US and European Union good manufacturing practices (GMPs) require microbial surface monitoring in pharmaceutical cleanrooms.
The following is an overview of the requirements and detection methods:
Air requirements
- Total particulates (inert or nonviables, plus viables)
- Particle counting (0.5 µm in diameter or larger)
- Viables (bacterial, fungal cells, spores)
- Settle plates (passive air sampling)
- Air samplers (active air sampling)
Surface requirements (viables)
- Facilities (including walls, floors, etc.) and instruments
- Contact plates
- Swabs
- Personnel (gloves, garment, etc.)
In addition, Section 18 of the EU aseptic manufacturing GMP document (Eudralex volume 4, Annex 1, 2008) establishes the following guidance:
- Where aseptic operations are performed, frequent monitoring using methods such as settle plates, volumetric air and surface sampling (e.g. swabs and contact plates)
- Surfaces and personnel should be monitored after critical operations.
- Additional microbiological monitoring is also required outside production operations, (e.g. after validation of systems, cleaning and sanitisation)
Similarly, the US Federal Drug Administration (FDA) GMP guidelines (Chapter V, section C, Monitoring Program) establishes the following:
- Personnel can significantly affect the quality of the environment in which the sterile product is processed. A vigilant and responsive personnel monitoring programme should be established.
- Monitoring should be accomplished by obtaining surface samples of each operator’s gloves on a daily basis, or in association with each lot.
- This sampling should be accompanied by an appropriate sampling frequency for other strategically selected locations of the gown.
Microbial surface monitoring
Some surface contamination monitoring methods detect microbial contamination from sources such as surfaces, garments, gloves and equipment based on measurements of oxygen depletion from incubation.
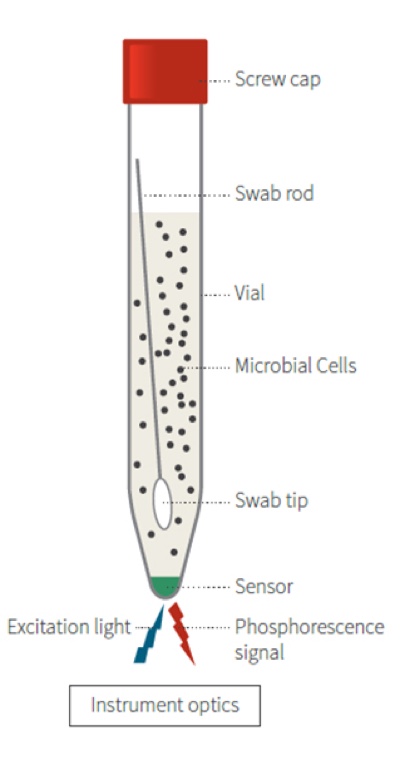
The technology is based on the growth of the contaminating microbial culture and follows the process oulined below:
- Bacteria grow in a sample (incubated) and in doing so consume dissolved O2.
- The fluorescent O2 sensitive polymer probe inside the vial reacts to the O2 depletion.
- The measure of bacterial O2 consumption equates to microbial load.
This is based on the following principles:
- When O2 concentration increases, it causes a reversible decrease in the phosphorescence signal.
- Inversely, when microbial respiration is active, the oxygen concentration in the media decreases and the sensor produces a larger phosphorescence signal.
- If the sample has microbial contamination, the time that it takes the phosphorescent signal to cross a predetermined threshold is the time-to- result (TTR). The greater the initial microbial load, the faster the result.
O2 = Phosphorescence
Testing and results
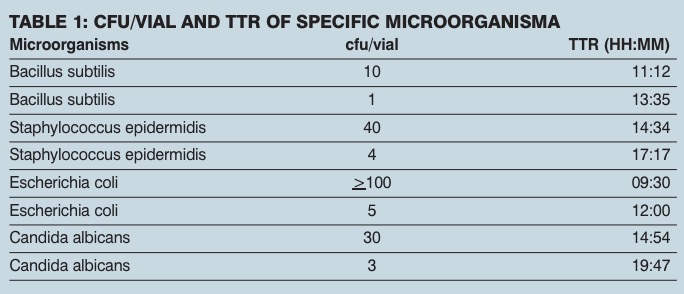
Testing was carried out to evaluate the feasibility of using an oxygen depletion-based method as a rapid microbiology assay. The TTR for a large number of bacterial, yeast and mould cultures tested was less than 24 hours. The method was capable of detecting less than 5 cfu/ml with a TTR of about 12 hours (see table 1) leading to faster microbiological evaluation of pharmaceutical samples compared with traditional methodology.
Linearity was observed in a broad range of microorganism concentrations (1x100 to 1x105 cells per tube) and linear regression analysis showed that, in all cases, the square of the correlation coefficient (r2) values were greater than or equal to 0.95.
Results were automatically read, eliminating human reading subjectivity and showing a high degree of precision. In addition, validation results show that the method has good sensitivity and good limit of detection (LOD) parameters similar to those obtained with traditional methods.
RMM technologies for surface contamination detection are capable of being non-destructive, ready-to-use disposable testing methods with simple designs. Automatic detection level results were as low as 1 cfu, allowing presence/absence determination within 24 hours of sample collection.
Tests demonstrated RMM technology is reliable, sensitive and equivalent to standard compendia tests. A typical dilution series of a microbial species demonstrated excellent linearity across a broad range of cell counts.
The rapid microbial method presented here represents a valid alternative application for the microbial monitoring of surface pharmaceutical samples.
Bibliography
- Parenteral Drug Association (PDA): www.pda.org
- Food and Drug Administration (FDA): www.fda.gov
- United States Pharmacopoeia (USP): www.usp.org
- Particle Measuring Systems: www.pmeasuring.com
- European Pharmacopoeia, EP 5.1.6 Alternative Methods for Control of Microbiological Quality
- PDA Journal of Pharmaceutical Science and Technology. May/June 2008. 62, 3. 191-199.
- USP Chapter <1116> Microbial Monitoring in Aseptic Environment





