Before embarking on a discussion about flexible material types that are suitable for injectable or parenteral drugs, it’s important to understand the key attributes that are typically required and the growth trends driving this sector of the marketplace.
Recent research conducted by consultancy firm IQVIA shows strong growth in speciality medicines that target chronic, complex or rare diseases: cancer, autoimmune, HIV, etc. There is also an uptake in biological drugs versus chemicals. The research showed that although oral solid dosage forms still dominate the marketplace, injectable medicines are growing at a more rapid pace.
The study continued to point out that increases in healthcare costs are prompting a shift from hospital to home-based care. This, in turn, is driving packaging innovation that facilitates the ability of the patient to self-administer medications.
Because many of these therapies are delivered by injection, medical device dosage forms have evolved from vial/syringe combinations to prefilled syringes to flexible reservoirs in wearable devices. The drivers are lower cost, reduced exposure to nosocomial infections and patient preference for independence.
Flexible packaging selection criteria
To select the right materials for injectable drugs, several attributes need to be considered. The most important ones include shelf life, temperature, moisture loss/ingress, oxygen ingress, light (photostability and transparency), sterility/microbial barrier and extractables/leachables/sorption. Let’s look at these individually.Shelf life: desired shelf life will play a significant role in what packaging structure is chosen for the application. How will the material protect the contents with the passage of time? The longer the desired shelf life, the higher the material expectations.
Temperature: how sensitive is the drug’s efficacy to temperature variations? It’s important to take into consideration environmental and home usage scenarios to understand how to engineer the packaging so that the product is not compromised. Further, since many temperature-sensitive drugs are transported via cold chain distribution, it is important that the films be resistant to “cold cracking.”
Moisture loss: if the packaging structure selected does not provide sufficient moisture barrier for the liquid drug it contains, then that aqueous solution will start to evaporate, thereby changing the concentration of active ingredient. On the other hand, if the package contains a powder that needs to be reconstituted before use, that also requires protection against moisture ingress.
Light: some active ingredients are sensitive to light. However, visual inspection requirements frequently specify the structure to be transparent, putting the two objectives at cross purposes. The ability to conduct a visual inspection is especially important for liquid injectables. The goal is to make sure there are no visible particulates that would compromise injection.
One workaround that can be considered is an aluminium foil outer package that protects against light but can be removed so that the contents are inspected in their primary transparent packaging immediately prior to use.
Sterility and microbial barrier: Typically, these liquid drug packages are sterilised after filling/sealing. The package needs to have a hermetic seal to prevent leakage and contamination. The structure also needs to be puncture resistant and not degrade during the selected sterilisation process.
Drug/material interaction: the relationship between drug and packaging material needs to be evaluated so that there is no unwanted interaction that would impact efficacy.
Packaging structures
If you look at key requirements such as stability, sterility and shelf life, selecting a packaging structure for pharma applications requires consideration and expertise. Although glass containers can provide many of the desired attributes, their heavy-weight and breakage susceptibility make the material less than ideal for use with these products.
On the plastics side of the material spectrum, drug developers tend to gravitate towards either multilayer films or injection moulded containers to deliver key performance objectives.
Rigid containers can typically fulfil barrier requirements more readily due to increased wall thickness inherent in the process.
Flexible structures, on the other hand, enable a higher level of design flexibility and functionality. For example, drugs need to flow consistently through the delivery system without air being trapped. Small reservoir pouches for wearables and IV bags that are easy to empty are just two examples of how flexible materials are used to facilitate proper dosing.
Materials selected for injectables have to provide excellent stability to support pharmaceutical efficacy. The most commonly used are polyolefins, which include polyethylene (PE), polypropylene (PP) and cyclic olefins, such as Cyclic olefin copolymer (COC) and Cyclo-olefin polymers (COP). Polyolefins have been widely evaluated and used for pharma applications for many years. Resin producers have developed dedicated resin grades with high purity to meet requirements.
The end result is that drug manufacturers have a significant amount of experience and documentation with using polyolefins as the pharmaceutical contact layer.
Packaging structures
If you look at key requirements such as stability, sterility and shelf life, selecting a packaging structure for pharma applications requires consideration and expertise. Although glass containers can provide many of the desired attributes, their heavy-weight and breakage susceptibility make the material less than ideal for use with these products.
On the plastics side of the material spectrum, drug developers tend to gravitate towards either multilayer films or injection moulded containers to deliver key performance objectives. Rigid containers can typically fulfil barrier requirements more readily due to increased wall thickness inherent in the process.
Flexible structures, on the other hand, enable a higher level of design flexibility and functionality. For example, drugs need to flow consistently through the delivery system without air being trapped. Small reservoir pouches for wearables and IV bags that are easy to empty are just two examples of how flexible materials are used to facilitate proper dosing.
Materials selected for injectables have to provide excellent stability to support pharmaceutical efficacy. The most commonly used are polyolefins, which include polyethylene (PE), polypropylene (PP) and cyclic olefins, such as Cyclic olefin copolymer (COC) and Cyclo-olefin polymers (COP). Polyolefins have been widely evaluated and used for pharma applications for many years. Resin producers have developed dedicated resin grades with high purity to meet requirements.
The end result is that drug manufacturers have a significant amount of experience and documentation with using polyolefins as the pharmaceutical contact layer.
Specific attributes
Here are some specific attributes for the more commonly used polyolefins for drug applications:
Polyethylene:
- Stable, well-known material
- Good resistance to radiation sterilisation
- Healthcare grades (USP & EP compliant) available from several suppliers
Polypropylene
- High-temperature resistance (suitable for steam sterilisation)
- Property versatility (random copolymers, homopolymers)
- Healthcare grades (USP & EP compliant) available from several suppliers
Cyclic olefin (co)polymer (COC/COP)
- A highly inert, high-purity material
- Meet requirements of USP & EP, and ISO 10993 biocompatibility
- The preferred material for vials and prefilled syringes as a replacement to glass (non-breaking, low protein adsorption)
- High transparency
- High chemical resistance
Demanding applications
Cyclic olefins are now being used for more demanding applications when both PE and PP are not inert enough. The disadvantage of any olefin polymer on its own is that it typically does not provide sufficient oxygen and moisture barrier; environmental moisture, as well as evaporation.
This is where polychlorotrifluoroethylene (PCTFE), commonly known by the brand name Aclar, comes into play: a layer of Aclar can be added to the polyolefin layer to provide significant moisture barrier properties while maintaining transparency. The only other material that can provide even more barrier at the same thickness is aluminium foil, but the desired transparency is lost.
Additional attributes of the PCTFE Aclar include:
- Flexible thermoplastic film
- Ultra-high moisture barrier
- Chemically very stable and inert
- Low leachables/extractables
- Sterilisation resistant
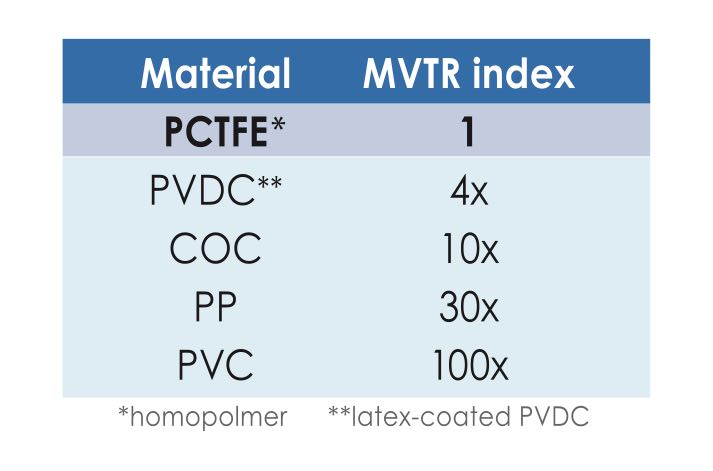
Table 1
Where moisture barrier is concerned, you would need 100 units of PVC to achieve the same effect to one unit of thickness of PCTFE (Aclar). View table 1 to see how the numbers compare. The goal with flexible structures is getting the best barrier at the lowest possible thickness.
Film lamination vs. co-extrusion coating
Adhesive lamination is a more traditional technique used to bond films together. Individual film layers are combined with adhesives to create the final structure.
Adhesive lamination offers significant material choice flexibility. However, the downside is that you have to form the individual films, then use adhesives to bond them, which adds another step to the process. In addition, adhesives are usually dispersions of small molecules that eventually cross-link into larger polymer networks, but with the potential of residual small molecules that can migrate out.
In the co-extrusion process, the multilayer film is created in one step. In this process, there are no adhesives involved, but "tie resins": large molecules, created by grafting functional bonding groups onto a polyolefin backbone, and they will form a chemical bond between the layers.
Co-extrusion is the preferred process for injectable products due to low leaching attributes.
Here is a typical structure using either process:
- Polyolefin contact layer
- Tie resin
- Barrier layer
- Tie resin
- Polyolefin outer layer
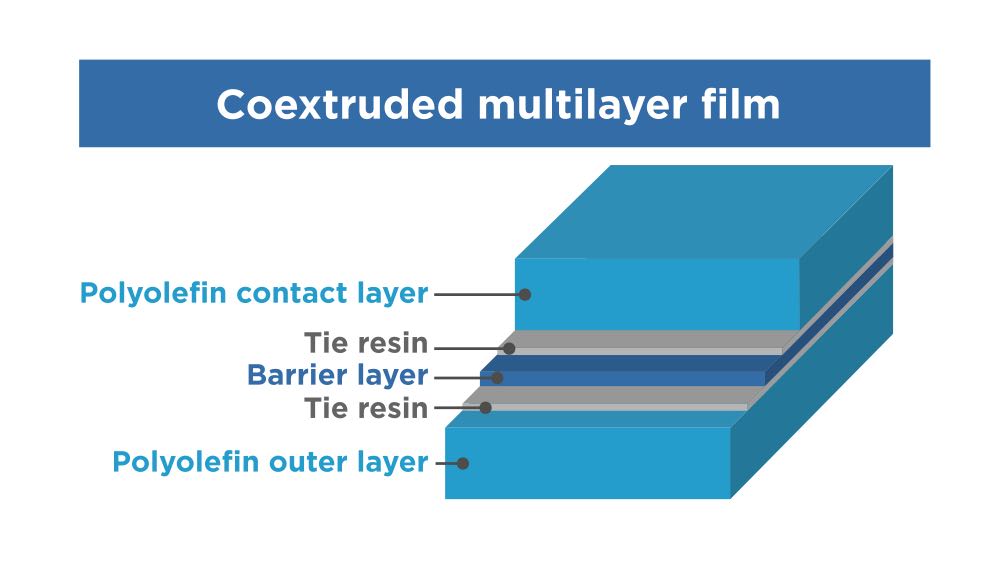
Polyolefin cohesive multilayer film
Cleanroom film manufacturing
Both laminated and coextruded films for injectable applications are produced in a cleanroom, which limits the number of particles in the air. The objective is to minimise the chance that the film will be contaminated. For pharmaceutical film manufacturing, a Class 8 cleanroom is typically required.
A film roll is typically double-bagged before it leaves the cleanroom for transport. This enables the converter or pharmaceutical manufacturer to remove the outer bag before bringing it into their cleanroom, where the second bag is removed prior to converting into pouches or other structures.
The sterilisation technique used for these products will depend on the specific drug type and the type of packaging that houses it. Most widely used is radiation (gamma or e-beam) followed by ethylene oxide (usually for packaging materials before drugs are filled), and steam, which is used to sterilise plastic structures with higher temperature resistance; radiation sterilisation usually occurs at ambient temperatures.
It’s also important to consider the radiation technique in conjunction with the impact on the plastic's properties. For example, some plastics can be prone to yellowing or to becoming brittle when subjected to a specific sterilisation process.
Closure systems selected for these drug delivery packages need to provide enough protection so that shelf life is not compromised and that there is no leakage in transport.
There are a variety of closure types that are used ranging from elastomers that allow needle access to connectors that enable the drug to be dosed. Film manufacturers tend to work closely together with pharmaceutical companies to develop a custom-built structure that can meet performance requirements as well as easily accommodate the preferred closure device or system.
A look to the future
As we move into the next decade with an increasingly ageing population, more attention will be given to improving drug delivery methods, patient comfort and ease of use. The trend is coupled with the introduction of new connected devices that transmit data to physicians about patient compliance, dosage and more. These challenges will continue to push the boundaries of plastic packaging structures for this market segment.
N.B. This article is featured in the January 2019 issue of Cleanroom Technology. The digital edition is available now.
