This article delves into the CAR T-cell genetic modification process, the crucial role contamination control plays, and the critical role of Personal Protective Equipment (PPE) in maintaining a secure environment.
The CAR T-Cell Genetic Modification Process
1. Collection of T Cells:
- The CAR T-cell therapy process begins with the collection of a patient's T cells through leukapheresis, a procedure similar to blood donation.
- Leukapheresis separates T cells from other blood components, ensuring a high concentration of the target cells.
- Ensuring a clean and sterile collection environment is essential to prevent contamination, as any compromise at the start of the process can impact the effectiveness of the therapy.
2. Isolation and Activation:
- Once collected, the T cells are isolated and activated in the laboratory. They are then cultured to ensure they are in an active state and ready for modification.
3. Genetic Modification:
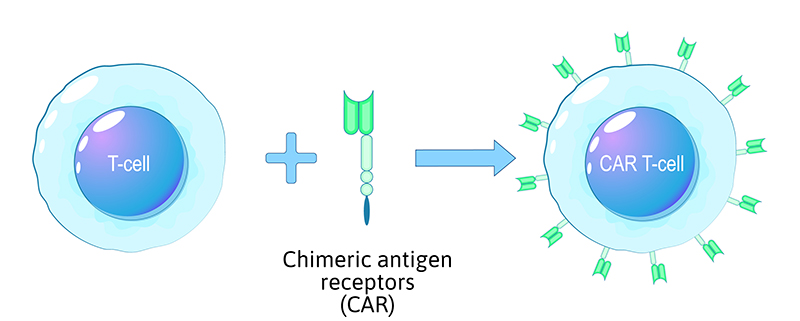
- Genetic modification is a critical step in the CAR T-cell process. Scientists introduce a new genetic sequence into the T cells to enable them to express the Chimeric Antigen Receptor (CAR).
- This CAR is engineered to recognise specific antigens found on the surface of cancer cells.
- Lentiviral or retroviral vectors are typically used to deliver the CAR gene into the T cells' genome. Strict contamination control ensures the integrity of the genetic modification process.
4. Expansion:
- After genetic modification, the CAR T cells are expanded in culture to create a larger population of these specialised immune cells. This step ensures there are enough CAR T cells to mount an effective immune response.
5. Quality Control:
- Rigorous quality control measures are in place to ensure that the modified T cells are safe and effective. This includes assessing the purity, viability, and functionality of the CAR T cells.
6. Infusion into the Patient:
- The final step involves infusing the modified CAR T cells back into the patient's bloodstream. This infusion marks the beginning of the therapeutic phase, where CAR T cells recognise and attack cancer cells with the targeted antigen.
Contamination Control Measures
Effective contamination control is imperative throughout the CAR T-cell genetic modification process to maintain the integrity of the therapy and ensure the safety of both patients and laboratory personnel. Key contamination control measures include:
- Sterile Techniques: Laboratory personnel must strictly adhere to sterile techniques, including handwashing, gowning, and gloving, to ensure that all equipment and materials used are sterile.
- Biological Safety Cabinets (BSCs): Genetic modification of cells takes place within Class II Biological Safety Cabinets, which provide a controlled and sterile environment. These cabinets help contain potential contaminants and protect laboratory workers.
- Cleanroom Facilities: CAR T-cell production often occurs in ISO-class cleanroom facilities, which are specifically designed to maintain high levels of cleanliness and control particulate contamination.
- Isolation Barriers: Physical isolation barriers, such as laminar flow hoods and closed systems, are used to prevent contamination during cell manipulation and culture processes.
- Routine Monitoring: Regular monitoring of laboratory environments for air quality, surface contamination, and microbial presence is essential to identify and address potential sources of contamination promptly. Common monitoring tools include particle counters and microbial samplers.
- Waste Disposal: Proper disposal of waste materials, including potentially contaminated equipment and biological waste, is crucial to prevent contamination. The disposal of contaminated materials, including sharps, is vital to prevent any accidental exposure to personnel.
Personal Protective Equipment (PPE) in the CAR T-Cell Genetic Modification Process
Given the potential risks associated with working with genetically modified cells and viruses, the use of appropriate PPE is paramount to safeguard the health and safety of laboratory personnel involved in the CAR T-cell therapy process. The following are key PPE components used during various stages:
- Lab Coats and Coveralls: Lab personnel should wear disposable lab coats or coveralls to minimise the risk of contamination. Ansell’s range of sterile lab coats and coveralls are strategically folded to aid aseptic donning and are manufactured from lightweight, antistatic, low-linting material. Learn more about sterile protective clothing.
- Gloves: Disposable sterile gloves are essential to protect hands when handling potentially infectious materials, ensuring that any contact with skin or mucous membranes is avoided. Gloves are also required to protect the critical modified T-cells from contamination. Ansell’s wide selection of sterile gloves is fully validated to achieve SAL 10-6 and is available in a range of materials and thicknesses to provide the desired dexterity and tactility to perform intricate procedures. Skin-friendly options are available to significantly reduce the risk of Type IV allergies. The 73 series of glove styles, tested for biocompatibility according to ISO 10993-11 Biological Evaluation Test for Systemic Toxicity, are designed to ensure the preservation and integrity of living cells during use. Explore the sterile glove range.
- Eye Protection: Safety goggles protect the eyes from potential splashes or aerosols that may contain genetically modified materials. Ansell’s sterile single-use goggles comply with EN 166 certification for assured personal eye protection, have indirect ventilation systems to reduce the risk of contamination entering the controlled environment, and have anti-scratch and anti-fog lenses for clear and undistorted vision. Autoclavable versions are also available. Learn more about sterile goggles.
- Facemasks: Depending on the level of risk, respiratory protection may be necessary, especially when working with lentiviral or retroviral vectors that can be aerosolised. Ansell offers sterile looped or tie-on facemasks with high particle and bacterial filtration efficiency (BFE) and cleanroom-compatible materials to reduce the risk of contamination. View sterile facemasks.
- Sleeve Covers and Overboots: Disposable sterile overboots help prevent any contamination from footwear, and disposable sterile sleeve covers offer an additional layer of protection to the wearer and at the point close to the product. Check out sterile overboots and sleeve covers.
- Double-Gloving: In some cases, double-gloving can provide an extra barrier against contamination and is recommended when handling hazardous chemicals and materials.
Conclusion
The CAR T-cell genetic modification process represents a groundbreaking approach to cancer treatment. However, its success depends on meticulous contamination control measures at every stage—from T-cell collection to infusion into the patient. These measures ensure the safety of laboratory personnel, the viability of the modified T-cells, and the effectiveness of the therapy.
By adhering to stringent contamination control protocols and using the appropriate PPE, researchers and healthcare professionals can harness the full potential of CAR T-cell therapy to combat cancer and improve patient outcomes while minimising the risk of contamination-related issues.

