Profile
What we do
AM develops, manufactures and distributes state-of-the-art products and services for contamination control in cleanroom.
AM PRODUCTION
AM Production is the business unit specialising in the creation of cleanroom contamination control products and technologies, characterised by excellence and innovation in production processes and solutions offered. Each product is manufactured with rigorous attention to risk analysis and regulatory requirements, ensuring maximum operational safety and full compliance.
AM's production boasts 4 patents and 25 registered trademarks, the result of 35 years of activity and constant commitment to research and development of solutions suitable for the Life Sciences market.
The production environments, 1000 sqm of Grade C Operational cleanroom and an innovative technology hub, ensure production meets the regulatory requirements of the Life Sciences industry.
The Quality Management System verifies every step of the processes, creating synergy between operating methods, goals and results shared with our customers.
Pharmaclean®
Pharmaclean®is AM's brand dedicated to the study, production and marketing of high-quality cleanroom consumables, representing the benchmark for those working in controlled environments.
Pharmaclean® stands out for its ability to offer tailored solutions that optimise efficiency, reduce risk, and ensure maximum compliance of operations in protected environments, where even the
invisible detail makes a difference.
Manufacturing sites in Italy specialise in Tyvek® items designed for packaging, sterilisation and material transfer processes in contamination-controlled environments.
- Pharmaclean® Tyvek® covers and bags
- Pharmaclean® Integra Tyvek® - PET/PP bags and reels
- Pharmaclean® Tyvek® - PET/PP bags and reels
Other lines:
- Cleaning tools and accessories
- Wipes
- Stationery for cleanroom
- Decontamination Mats
Zherox®

Zherox® is AM's brand specializing in environmental biodecontamination systems, synonymous with innovation, effectiveness and safety for operators.
Thanks to its unique Electro-activated Hydrogen Peroxide (HPE) technology, which uses a solution with less than 8 %H2O2, Zherox® ensures uniform and effective biodecontamination on all surfaces, including hard-to-reach areas, setting new standards in the industry.
The versatility of the system allows its application in different solutions-mobile, stationary, portable and pass-box systems-to meet specific operational needs.
- Zherox® mobile systems for environmental biodecontamination (available in full remote management through tablet PC till 20 units and stand alone version)
- Zherox® b-pack, portable system, for surface biodeconmtamination
- Zherox® fixed system
- Zherox® MyBox, pass-box with integrated biodecontamination system
Gloves for isolators
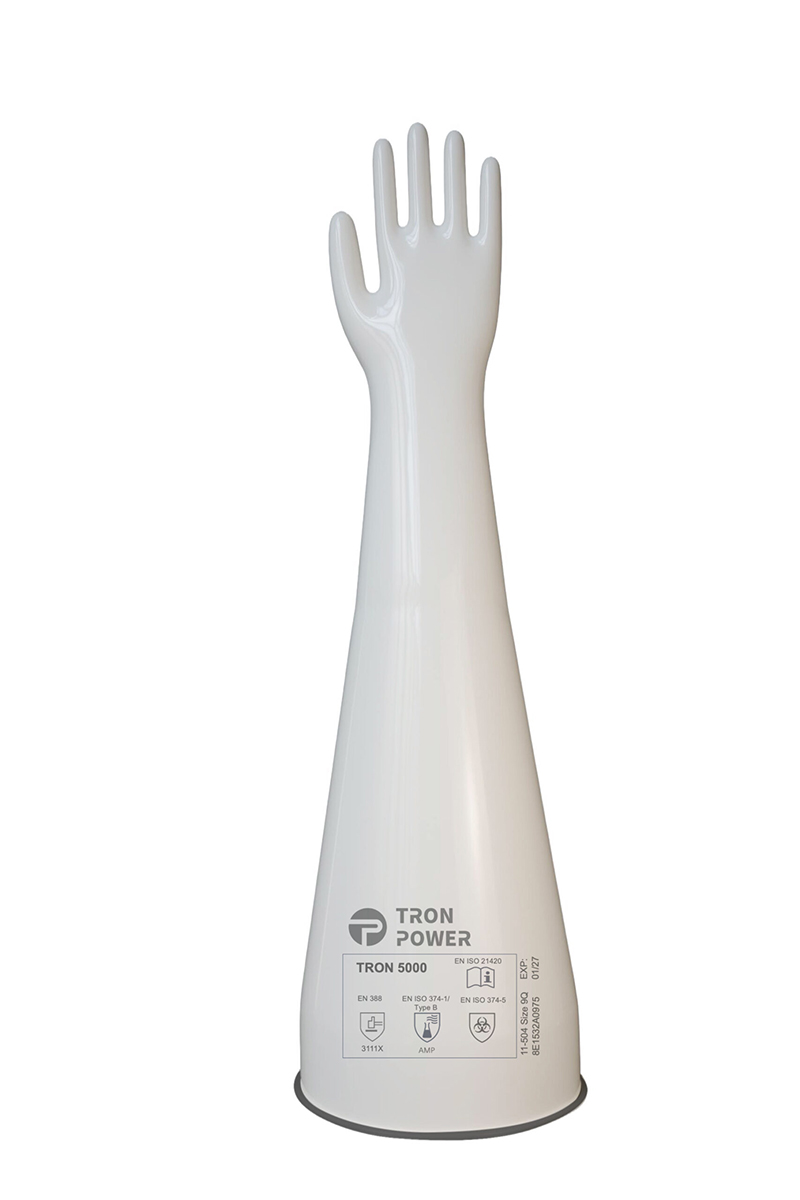
Tron Power isolator gloves, distributed by AM, stand out for comfort, quality and safety. Ergonomic and comfortable, they enable high agility and precision in manual operations while providing maximum protection for the operator and the product. We offer a complete line of gloves and sleeves for isolators and RABS, designed to fit every environment and process.
The line also covers PuretekTM gloves, cleaned and sterilised, for pharmaceutical critical environments.
Available in different lengths, materials, diameters, thicknesses and sizes, our range can meet all kinds of needs.
