As the COVID-19 virus spread around the world causing critical mask shortages, Comecer decided to study a solution which would put to good use its experience decontaminating the isolators it manufactures for pharmaceutical companies.
The result is DeconBox, a mobile, purpose-built, VPHP bio-decontamination system that allows FFP2 and FFP3 9 Filtering FacePiece masks to be reused. The system is suited for hospitals and other organisations that must provide masks to their employees. Using Deconbox doesn't just eliminate supply issues, it also reduces the environmental impact of discarding a large number of masks daily.
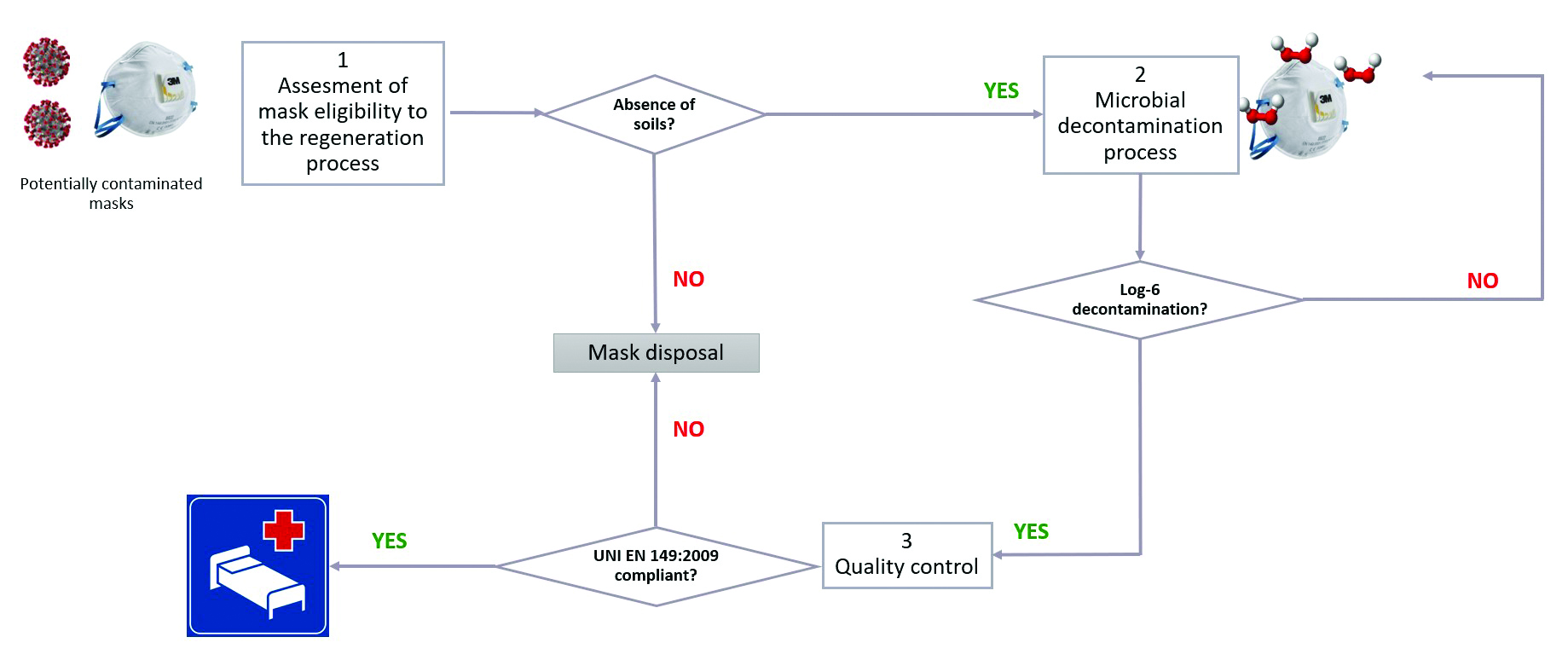
Figure 1: A generic operative process flow for Filtering FacePiece (FFP) mask regeneration
DeconBox is also cost-effective as savings start when an organisation uses more than a total of 200 masks per day (try the DeconBox Convenience Calculator). We shall be taking a look at the test methodology and the results behind the guaranteed mask decontamination.
As a starting point for our internal test, we considered a generic operative process flow for Filtering FacePiece (FFP) mask regeneration (Figure 1). In that context, DeconBox takes a role in the step dedicated to the microbial decontaminating process. However, this treatment could lead to several consequences impacting the success of the quality controls.
Colony Forming Unit (CFU) were used to estimate the number of viable bacteria
For this reason, our aim was to develop a Vapour-Phase-Hydrogen-Peroxide (VPHP) cycle associated to the DeconBox, compliant with the following three conditions:
- The cycle must repetitively guarantee a log-6 reduction of the analysed biological indicator.
- At the end of the cycle, the VPHP residues stuck into the mask matrix must be lower than 1 ppm.
- The filtering performance of the masks must be preserved to comply with the UNI EN 149:2001 + A1:2009 legislation.
Mask eligibility
Previous studies showed how blood and other organic soils could impair the efficacy of VPHP-driven bioburden reduction in tissues and surfaces. We collected used FFP masks and only those without any trace of organic soils were selected for our internal test. Two different types of FFP mask were analysed: 3M Aura 9332+ and Honeywell 2211 as models for FFP3 and FFP2, respectively.
Customised biological indicator
The Tier classification system was introduced by the FDA to define the level of bioburden reduction required during the microbial decontamination process of FFP mask and respirators. Tier 1 level claims the verified ≥ log-6 spore reduction of the most resistant spore for the proposed process. Our ultimate goal was to develop a VPHP-based decontaminating process for FFP masks able to assure a Tier 1 level.
Spores of the strain ATCC 7953 of Geobacillus stearothermophilus are widely exploited in validation processes of pharmaceutical and industrial VPHP decontamination cycles due to their proven resistance to hydrogen peroxide. We selected this specific strain of spores as a biological indicator (BI) for the bioburden reduction level required for Tier 1 class.
Commercially available BIs take advantage of a monolayer of a certified amount of spores spotted on disks of common industrial materials, such as 316 stainless steel and polyethylene. However, due to the 3D structural complexity of FFP masks given by the multi-layer tissue, BIs able to simulate its interactions with microbial agents and sterilant are not currently available on the market. Therefore, we designed a customised BI for both the FFP masks under analysis with the purpose to address the achievement of the log-6 decontamination of the previously mentioned ATCC 7953 spores through VPHP.
Mask pieces were cut, sterilised and then an average of 1.5*106 spores (target loading of 1.5x106 CFU per 1 sqcm) (Mesalab) was spotted on each piece. This system avoids potential cross-contamination during the handling of BIs at the end of the decontamination cycle.
The treated BIs were subsequently incubated in Tryptic Soy Broth (TSB) modified with pH indicator at 57.5°C, allowing the rapid discrimination between active spores and inactivated spores directly from the colour variation of the broth.
VPHP Decontamination cycle
Based on tests performed on the DeconBox and results obtained with the previously described BI, we selected a unique VPHP cycle appropriate for most of the FFP masks present on the market.
The VPHP cycle is characterised by the parameters in table 1. The overall cycle lasts 7.5 hours, with a total INTEROX H2O2 35% consumption of 150 g. Moreover, the decontaminating efficacy of the cycle was proven at different external temperatures, ranging from 23°C to 32°C.
Assessment of bioburden reduction
To officially validate the VPHP cycle from a microbiological point of view, we established a collaboration with the microbiology department at the Università di Modena.
Due to the 3D complexity of FFP masks, no BIs are currently available to simulate microbial interactions
Besides the preparation of the same BI, the microbiological laboratory team also prepared BIs containing spotted titres of Cox B5 and HumanCoronaVirus-OC43 (HCoV - OC43) viruses, respectively selected for the high resistance to VPHP and the phylogenetic proximity to the well-known SARS-CoV-2.

Figure 2: Tryptic Soy Broth modified with pH indicator at 57.5°C for rapid discrimination between active and inactivated spores
Once BIs have been collected at the end of the VPHP cycle, they were incubated in Tryptic Soy Broth and their growth analysed by the OD600 spectrometric technique. The impact of the VPHP on the Geobacillus stearothermophilus spores was further addressed by comparing the bacterial viability after the treatment (referred to as # BI) with respect to an untreated sample (ctrl +). Colony Forming Unit (CFU) were used to estimate the number of viable bacteria in a sample; in this case, CFU is relative to the non-inactivated spores able to give rise viable colonies. Thus, we used the concentration of CFU within the culture broth to directly quantify the effect of the VPHP cycle. Since the starting population of spores was 1.5x105, a log-6 reduction was obtained through the decontaminating cycle.
Similar results were achieved with the BIs containing the viruses. The plaque assay was also set up to experimentally calculate the magnitude of decontamination expressed in log. In both viruses, we observed a 100% reduction of the initial viral titre.
VPHP residues
A crucial point in the overall process of FFP mask regeneration is represented by the removal of potential hydrogen peroxide residual molecules stuck in the mask matrix.

Figure 3: DeconBox, a mobile, purpose-built, VPHP bio-decontamination system that allows FFP2 and FFP3 masks to be reused
In fact, according the Occupational Safety and Health Administration (OSHA) definition, the threshold limit value (TLV) for an operator exposed for 8 hours to VPHP is represented by 1 part-per-million (ppm), or the equivalent 1.4 mg/m3. So we designed an experimental apparatus able to generate a modulable air flux and, in parallel, measure the VPHP residues in ppm.
The resulting air flux was directed from the outer to the inner side of the mask to simulate the most realistic condition of H2O2 inhalation by operators. It was also set at 10 l/min to simulate the mean air flux of human breaths at rest and represents the most stressful experimental condition. Taking advantage of the experimental apparatus, we measured the VPHP residues released by the mask matrix under the 10 l/min air flux after 2, 3 and 4 hours of aeration 2 (Table 1), respectively.
We found that 4 hours represent the safety duration of the aeration 2 required for decreasing the H2O2 residues far below the 1 ppm TLV.
Conservation of performance
The third and last point essential for success is assuring that the native filtering performances of the mask are not impaired at all by the decontamination treatment. To demonstrate the non-invasiveness of our process, we commissioned two different tests on the tested FFP3 mask model, as governed by the EN 149:2001 + A1:2009 legislation.
A crucial point in the overall process is removal of potential hydrogen peroxide residual molecules stuck in the mask matrix
The first test is based on the measurement of the penetration yield of an emulsion of paraffin oil fluxed through the mask. Since the testing oil emulsion is characterised by droplets with an average of 0.3 µm in diameter and the filtration performances of the masks are claimed on that specific particle size, the percentage of penetration of the oil across the mask tissue represents an indirect valuable measurement of the filtration efficiency. Experimental parameters were found to be in accordance with the EN 149:2001 legislation.
Moreover, the VPHP-regeneration treatment does not significantly impair the filtration performance. In fact, the mean penetration percentage of the treated mask is statistically comparable with the un-treated control and far below the 1% threshold of paraffin oil penetration.
The second test aims to analyse the resistance that the FFP mask physically oppose to the air fluxes related to breath. Concerning the analysis of the exhalation resistance, the VPHP-treated masks are characterised by comparable air pressure drops with respect to the un-treated controls. The same result has been obtained also for the test of inhalation resistance, performed at both 30 l/min and 95 l/min air fluxes.
The sum total of these test and analyses showed the validation of the DeconBox for use with FFP masks.
N.B. This article is featured in the September 2020 issue of Cleanroom Technology. The latest digital edition is available online.
