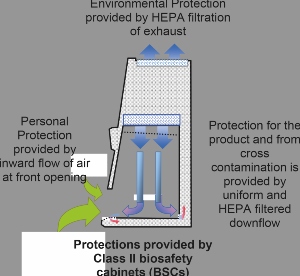
Current good manufacturing processes (cGMP) assure the quality and purity of drugs and rely on aseptic processing where terminal sterilisation is not feasible. While Class II biological safety cabinets (BSCs) have traditionally been used as a research and safety tool in a biological laboratory, they have immense promise in cGMP applications with minor adoptions to ensure necessary monitoring and measurement.
BSCs are designed to minimise hazards while working with low and moderate risk biological agents but are finding application within aseptic processing. By modifying their use to support cGMP requirement for documentation, cleaning, placement/process, monitoring, purpose and certification, BSCs can be a valuable tool in cGMP labs.
Real-time monitoring for containment of biological agents within a BSC is not feasible
Monitoring requirements for cGMP
BSCs were formally established in 1976, while aseptic processing became similarly established a decade later. Aseptic processing was used before 1987 and BSCs continued to develop after 1976, and while both address contamination of a work area, the mindsets and fundamental approaches within each discipline were established in separate times.
Another difference is in inspection and monitoring. cGMP requires continuous monitoring to verify environmental cleanliness during aseptic processing. However, real-time monitoring for the containment of biological agents within a BSC is not feasible. Instead, most BSCs rely on independent validation of performance on a representative type combined with periodic inspection (often called certification) of individual units in the field. Type testing assesses biological containment using an aerosol comprised of at least 100 million B. subtilis spores produced inside the cabinet and sampled for outside the cabinet. The biological nature of the challenge reflects the BSC purpose of providing increased biological safety.
Inspection and monitoring of cGMP and BSCs have both continuous and periodic components. Along with the periodic field certifications, BSCs constantly monitor window position and airflow with alarms for improper conditions. In addition to continuous process monitoring, aseptic processing in cGMP requires the periodic leak testing of HEPA filters.
BSCs use the same grade of HEPA filters and provide for the same integrity testing of those filters as other clean air devices. Type testing for BSCs includes performance verification for product protection and protection against cross contamination. When BSCs are subjected to air cleanliness testing, the work area meets or exceeds ISO Class 5 under normal use.
Annex 1 includes an air velocity guidance value that can only be met in BSCs by operating outside their validated parameters
When the aseptic processing requires a contained Grade A work area allowing manual intervention, the Class II BSC is an established solution with a long history of effectiveness. Given its proven performance, labs should consider how to bring BSCs into their cGMP workflow.
Design and initial qualification
Cleanliness can be validated and monitored with viable and non-viable particle counting. Validating and monitoring containment is more challenging. We can start by recognising the type testing and continue to use those validated inflow and downflow velocities. In the past, we may have taken a BSC with a strong history of performance and proceeded to disconnect the cabinet from that very history through modification of the structure and adjustment of velocities. For example, Annex 1 includes an air velocity guidance value of 0.36 – 0.54 m/s that can only be met in BSCs by operating outside their validated parameters.
BSC standards are protective of the geometry of the cabinet front opening and work area as changes could affect containment. NSF/ANSI 49 explicitly states major modifications to the cabinet will require tests for conformance to the standard. Examples of minor modifications not requiring additional testing include relocation of electrical outlets and petcocks. Bringing a cabinet into cGMP for its contained cleanliness and then modifying the features providing that contained cleanliness can actually reduce its effectiveness and would be inefficient.
Type testing lays the foundation, but we still need to validate performance in our process. BSC cleanliness at individual locations within the work area varies as the process moves through each step. Unidirectional airflow sweeping over and away is required while products are exposed. Once we define our process and the role of the BSC within the process, we will use airflow visualisation to demonstrate first air protection and unidirectional airflow that sweeps over and away from products exposed during processing.
Process monitoring and acceptable modifications
How do we monitor the contained Grade A work area within the cabinet? Do BSC standards prohibit required modifications for monitoring? Care must be taken, but there are a number of methods to be used to ensure cabinet performance meets the required applications. Air sampling tubes within the work area can monitor viable and non-viable particles without major modification. Placement of sampling tubes can be done such that the work area is changed in minor ways comparable to the movement of a vacuum valve from the side to the back. The change aligns with the description of a minor modification.
A recent addition to NSF/ANSI 49 provides for a “user-modified pass-through” to allow the safe and contained passage of wiring, cables, and tubing from the outside environment into the cabinet work area. This gives us a means to pass the cables and tubing into the cabinet that we may need for monitoring.
Some BSCs have gone beyond the simple on/off signal and provide voltage free contacts for air velocity alarms and window position alarms
Monitoring the window position and air velocities can serve as monitoring containment. This becomes especially promising through the common availability of voltage free contacts on BSCs. When volatile toxic chemicals are used, cabinets are connected to an external exhaust system. BSC designs support this functionality with voltage free contacts signalling cabinet fan operation. This allows the laboratory user to turn on the cabinet fan and signal an external damper to open or a roof exhaust to power up.
Some BSCs have gone beyond the simple on/off signal and provide voltage free contacts for air velocity alarms and window position alarms. If we have a cabinet with acceptable air flow and window position alarms, we can use these voltage free contacts to signal when improper airflow or window position compromises containment.
A traffic light system can be added with red, yellow, green to signal the acceptable state more clearly. We can integrate this with a switch on the cabinet where the operator signals the start and stop of aseptic processing. The signals can be recorded and used to document the maintenance of the work area during the particular work session.
Annex 1 also mentions the use of windows and cameras to allow observation of the process. Many European style cabinets have designs with transparent side panels. Cameras can be placed outside the BSC work area but viewing the process within the BSC without compromising the design.
Conclusion
Class II BSCs are useful strangers to aseptic processing within cGMP. However, with their long tradition of design and performance and Grade A environment, they are contained and can accommodate manual intervention. The disconnects to achieve cGMP use are addressable and include airflow visualisation for specific applications to augment type testing and accessorising the cabinets within the standard to meet our requirements.
Simple modifications include the use of low profile tubes for viable and non-viable particle sampling routed through approved user modified pass throughs. We should also select cabinets with monitoring for inflow, downflow and window position signalled through voltage-free contacts. Finally, providing a mechanism to signal the start and stop of the aseptic processing session enables clear signalling to the operators and documentation of the process.
With minor accommodations, Class II biosafety cabinets can be adopted into cGMP with no compromise to their performance.
In line with this, Thermo Fisher Scientific has recently introduced the Thermo Scientific 1500 Series Biological Safety Cabinet (BSC) to the Asian market. The new offering is designed to meet the demands of most laboratories and providing protection from biological hazards and contamination.
The 1500 Series BSC is the latest addition to Thermo Fisher Scientific’s innovative Class II BSC portfolio. It leverages Thermo Scientific SmartFlow Plus and DAVe Plus advanced airflow technologies to support automated airflow compensation and provides alerts for any out-of-spec conditions for added assurance.




