Cleanrooms are highly regulated environments that require the continuous monitoring of particle counts. In manufacturing cleanrooms, the safety and quality of products can be affected if too many particles enter the space. Setra’s CEMS provides a solution for environmental data, including particle counting, monitoring and recording needs required by various industries such as:
- Pharmaceuticals
- Medical device
- Healthcare
- Aerospace
- Semiconductor
- Nanotechnology
Setra CEMS Features
The goal of cleanroom monitoring is to assess the potential contamination risk of the product. Setra’s CEMS helps to continuously monitor an environment during the manufacturing process to simplify operations, minimize the risk of contamination, and improve the quality of products.
Setra’s CEMS is a single system that can monitor a diverse facility with multiple locations, providing instant environmental data access and sophisticated analysis from a desktop or tablet. Alarm notifications can be sent via text message and email. Instant, up-to-date reports and graphs provide easy access to proper documentation for regulatory requirements.
CEMS helps cleanrooms meat all the necessary regulatory requirements for ISO 14644, USP<797>, USP<800>, cGMP, EU Annex 1, GAMP, Joint Commission, FDA 21 CFR Part 11, EU Annex 11, and GAMP5. With simple networking and dependable remote access via the secure SetraCLOUD, users can save time and money with data exports and reporting features.
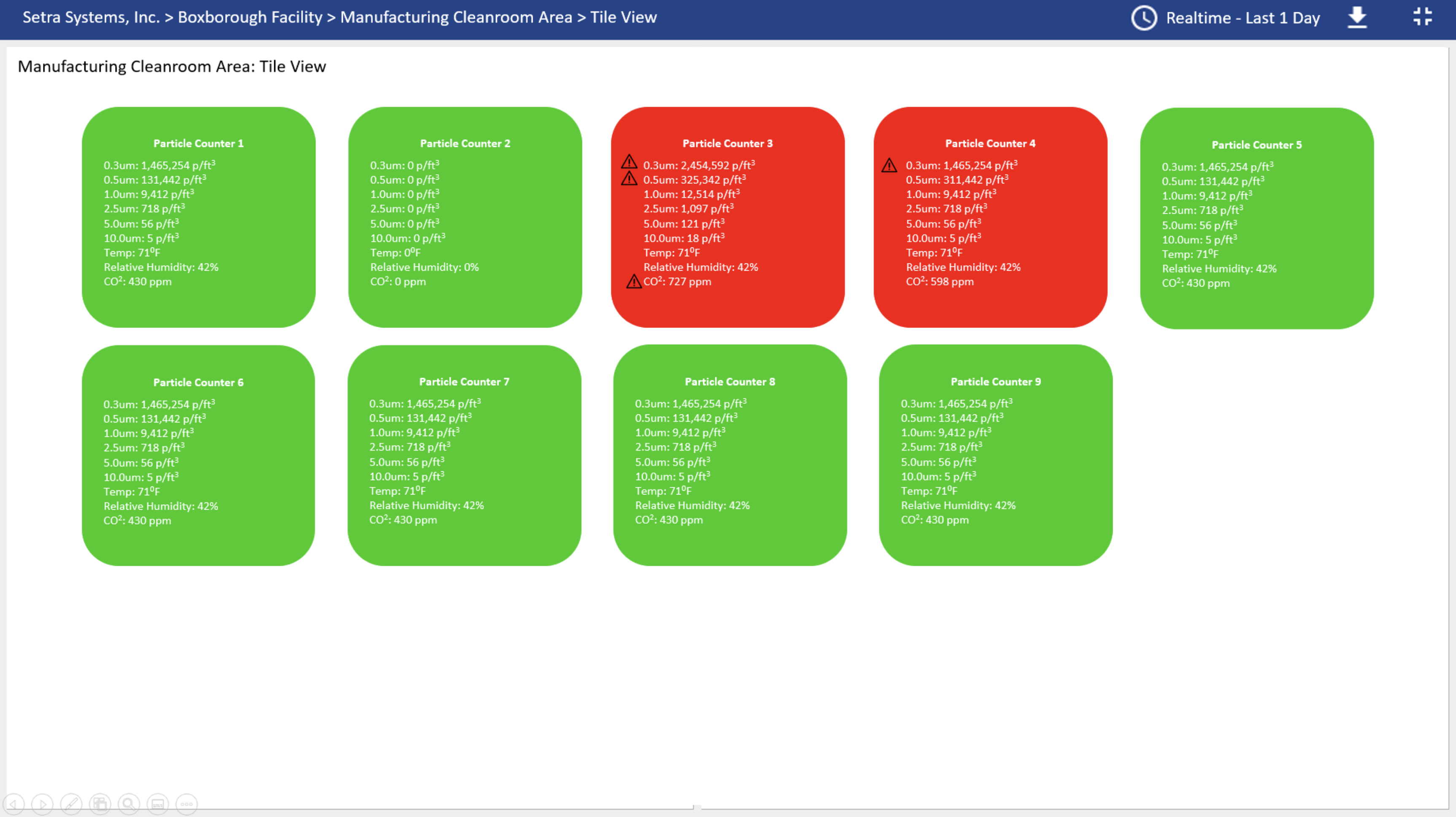
CEMS in Action
The below example is a customer who installed a total of 9 particle counters in their cleanroom facility. Spaces within their facility range from ISO 7 to ISO 9. Each particle counter is measuring concentration limits for particles ≥0.3µm, ≥0.5µm, ≥1.0µm, ≥5.0µm, ≥10.0µm, temperature (0F), % RH, and CO2.