Stainless steels with a high level of both chromium and nickel are generally used in pharmaceutical and other high-purity applications for processing liquids and gasses. Also known as austenitic stainless steel, the metal is very low in carbon. The 316L stainless grade includes molybdenum, which increases its resistance to corrosion.
One crucial physical aspect of stainless steel is that it naturally forms a passive film on its surface that protects the alloy. The quality of this passive film significantly affects the rate of corrosion and the potential for product contamination. Not only is it critical that the stainless-steel structure is manufactured correctly, fabricated and designed for the environment in which it will be subjected, but the metal itself must also be “passivated” to improve its corrosion resistance.
Passivation is the cleaning process that removes iron from the surface layer of the alloy. This process will form or improve the quality of the passive layer, preventing the metal from interacting with the chemicals or materials being processed.
Several specifications are available to guide an owner or contractor in assuring that the stainless steel surface is passivated correctly, such as those defined in ASTM A-967 and the ASME BPE Surface Finish requirements.
Passivation treatments over operational time should be performed regularly to maintain or restore the passive layer. If a surface has been subject to welding, surface finishing or other mechanical work, then treatment is required during maintenance or in the initial fabrication of the metal.
Passivation is generally performed with the use of specific acid solutions including nitric acid, citric acid or electropolishing with phosphoric acid. The resulting quality of the passive film formed and corrosion resistance is superior to the naturally occurring layer, with higher chromium levels and improved corrosion resistance.
The stainless-steel surface can be quantitatively evaluated by measuring the amount of chromium and iron at the surface. The ratio of chromium to iron in the surface passive film is an excellent indicator of how corrosion-resistant the surface will be. Chromium oxide is the main ingredient that resists corrosion and reducing the iron at the surface will, in turn, reduce the rate of rouge or iron oxide formation (rust).
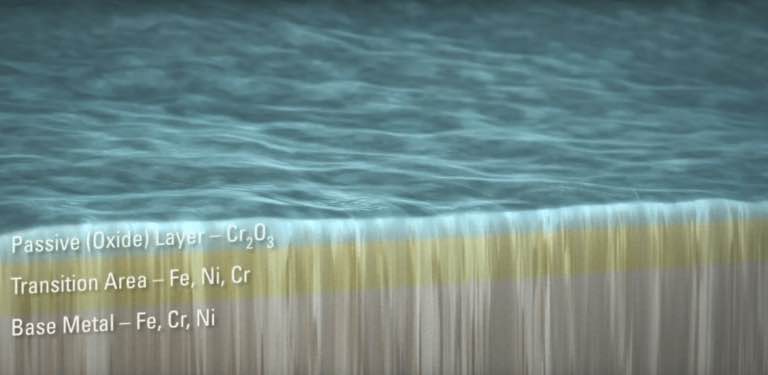
The ratio of chromium to iron in the surface passive film is an excellent indicator
for how corrosion-resistant the surface will be
Using phosphoric acid during electropolishing will generate a passive film of 1.1-1.3 Cr/Fe. Nitric acid produces a passive film of approximately 1.4 to 1.6 Cr/Fe. This ratio is the standard quality level of passive film that is specified in most industry standards, and it meets the requirements of ASTM A380 and A967. Citric acid also meets ASTM A967 and exceeds it with a Cr/Fe ratio typically of 1.7 to 2.0.
Although nitric acid provides a quality passive film, it is a hazardous material and produces hazardous waste. Nitric acid must be handled with great caution and use of proper PPE is vital. Citric acid with chelants produces an even better passive film with more chromium in the surface than the other treatment methods with the advantage of being a non-hazardous solution and waste. However, it does need to be employed at elevated temperatures (60-80 oC.) to be effective. Using citric acid results in a surface with approximately double the chromium concentration compared to natural passivation.
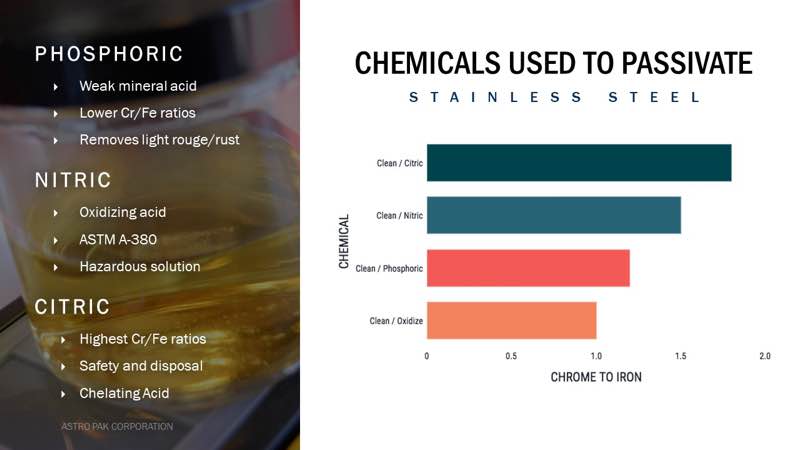
Phosphoric acid is very effective at removing light levels of rouge or corrosion products that form on the surface after periods of corrosion. However, it is not an effective passivation treatment since it does not produce a passive film that is equivalent to nitric acid.
Testing passivation treatment
Proper documentation and detailed record keeping is a critical aspect in high purity industries. Verification that a service provider's procedures meet quality standards is essential.
Industry organisations including ASTM, ASME BPE, and others provide adequate guidance in assuring that quality techniques, procedures and processes are verified by service contractors. Passivation procedures should meet the requirements of the specification and produce a good quality passive film.
There are many testing techniques to evaluate the efficacy of passivation treatment. These techniques can be divided into two basic categories: field testing and lab testing methods.
The field testing techniques use relatively simple solution and visual inspection methods such as the Ferroxyl test, copper sulphate test or rinse/dry cycling test.
While these tests show evidence of free iron on the surface, they don't evaluate the quality of the passive film, basically yielding a simple "passive" or "not passive" test result.

Use of more qualitative and quantitative lab testing methods will show the chemistry of the passive film, giving a more comprehensive evaluation of the surface treatment results.
Metallurgical test methods such as auger electron spectroscopy (AES), (X-ray photoelectron spectroscopy (XPS), or electrochemical testing analyse the surface chemistry and determine the level of chromium at the surface. These are the types of tests that are used to verify the quality and effectiveness of the proposed passivation treatments relative to industry specifications.
Biocontamination risk
A good passive film will result in a reduced level of corrosion as well as slow the formation of rouge.
Corrosive environments, especially those containing chlorides, will attack and degrade the passive film that protects the surface allowing iron oxide crystals to grow from the surface over time.
As this rouge builds on the surface, it can create particulate debris that is released into the process and product fluids. Corrosion will result in pitting of the surface and appear as roughness or a dull haze.
Rouge has been accepted in certain aspects of the industry and even said to be a protective layer. However, the truth is that rouge does not protect the surface. It's a result of corrosion and continues to build-up on the surface until it generates larger and larger particles that can interfere with the quality of the system fluids.
Controlling rouge formation, and eliminating rouge from critical fluid system surfaces, can be an important part of biocontamination prevention. An inspection of microscopic surface profiles reveals the increased roughness generated by corrosion and rouge.
A roughened and rouged surface facilitates the attachment of bacteria and their associated biofilms to the substrate. It is common for a rouged system to require more frequent sanitisation cycles to maintain bioburden control.

Removal of rouge from a system that is promoting bio-growth is critical to maintaining a bacteria-free surface. Depending on the size of the stainless-steel article in need of treatment, cleaning and passivation can be performed onsite or at a specialised facility. The type of service being performed can also dictate where it is conducted.
Passivation methods
Astro Pak performs several broad categories of passivation services in the field or at one of its facilities located across the US. Tank immersion is generally done at an Astro Pak site and is ideal for treating all fabricated surfaces simultaneously to render a uniform finish.
Circulation, where a chemical solution is circulated throughout a piping system, is typically done onsite and is particularly recommended for systems that will carry corrosive liquids.
Spray application can be done either in a facility or in the field where it has its advantages but requires proper disposal of the acid as well as personnel safety procedures.
Gel application is a manually-applied surface treatment involving brushed-on pastes or gels. It is ideal for spot treatments of welds and other intricate areas that require extra attention to detail.
Both cleaning and passivation are critical components for the initial preparation of an austenitic stainless steel surface used in high-purity applications. Stainless steel surfaces require an optimal passive film for corrosion resistance and a smooth surface for ease of maintaining cleanliness.
Removal of corrosion products and routine sanitisation treatments are required to maintain a bioburden-free surface in environments that host bacteria. Cleaned and quality passivated surfaces will provide long-term life of high purity equipment and surfaces.
N.B. This article is featured in the July 2019 issue of Cleanroom Technology. The latest digital edition is available online.
