Biopharmaceutical manufacturing is a complex process that involves acquiring, storing and using precise amounts of materials, many of which are dry powders packaged and shipped from suppliers in bulk amounts. These materials are typically handled by trained personnel in cleanroom packaging suites following strict protocols to prevent cross-contamination and ensure consistent product quality.
Preparing buffer and cell culture materials in a cleanroom setting can be labour-intensive and requires substantial investment in facilities and resources, as well as repeated quality assurance testing as bulk materials are subdivided for individual process runs. In addition, time-consuming cleaning and sterilisation is required between each batch of materials being processed.
New innovations in the packaging of raw materials can directly improve this process. Avantor’s J.T. Baker Direct Dispense packaging technology provides single-use, pre-weighed free-flowing delivery of products such as salts, buffers and other cell culture materials directly into production equipment. Materials delivered through the Direct Dispense system can essentially bypass the cleanroom — providing ways to make cleanroom operations more flexible and less of a potential bottleneck.
While pharmaceutical and biopharmaceutical producers are focused on quality, safety and drug efficacy, they are also continuously challenged to reduce costs and improve manufacturing efficiencies. One target for improvement is the cleanroom packaging operation, a necessary but labour-intensive process that can impede production flexibility and throughput.
Upstream biopharmaceutical processes consume raw materials such as cell culture media, carbohydrates, amino acids and buffers, typically supplied in powder form. The bioreactors and medium preparation tanks using these materials often operate around the clock: large-scale reactors with 10,000 L capacity can run continuously for up to 35 days, while newer generation single-use production systems, with multiple 2,000 L bioreactors are often configured to operate in overlapping sequences to achieve similar or greater productivity.
Until recently, it was common for most of these materials to be delivered in bulk amounts from suppliers, either in 100 kg drums with one or two plastic liners, or smaller cardboard boxes with plastic liners holding 50 kg. These bulk materials end up making at least two (and sometimes up to five) trips through the cleanroom packaging suite, as outlined below:
Initial receipt — to receive the material upon the shipment arrival, it needs to be sampled and properly identified. The bulk container is brought into a cleanroom packaging suite and opened to sample. This independently confirms via lab analysis that the quality, purity and characterisation match what was ordered. The bulk container is then closed and properly resealed for transport to the warehouse for later use. Some manufacturers also choose to take extra samples for additional characterisation tests or future reference.
Subdivision — once the material is ready for use, the container is brought back to the cleanroom environment where the appropriate amount is weighed and dispensed into interim packaging. If any material is unused from the original container, it often goes back to storage until needed and the subdivision process in the cleanroom is repeated.
Both the sampling upon receipt and the multiple subdivision steps all make use of the cleanroom and its personnel; for a 2,000 L bioreactor, a manufacturer may need to subdivide a 100 kg drum of material between two and five times.
Cleanroom processes
cleanroom facilities and personnel are some of the most tightly scheduled resources in the biopharmaceutical production environment. Given the round-the-clock operations at these companies, manufacturers need to strike an extremely careful balance between stringent QC and improving efficiency.
Consider the basic steps involved in conducting a standard subdivision of buffer materials:- The bulk container exterior is typically cleaned of any particulates and brought into the cleanroom by fully gowned personnel. This is typically done one container at a time, to eliminate cross-contamination risks
- Proper, step-by-step procedures need to be followed for opening each bulk container, hand-separating and weighing the material to be delivered to production
- Since the bulk container is open throughout subdivision, some biopharmaceutical producers conduct multiple lab analyses of these drums, each time a new quantity of material is removed, to confirm that no issues have occurred
- All devices and gloves used must fully comply with the cleanliness demands of the cleanroom and the work undertaken in the cleanroom. They must be cleaned, disinfected, or sterilised as appropriate for the type of work being done and the risk they pose for contamination
- On completion of subdivision, both the bulk material container and the container holding the production buffers must be sealed and the exteriors sanitised once more before they are delivered to the warehouse or the bioreactors
- Once this material is removed, full decontamination procedures need to be followed before the next material for subdivision is brought into the cleanroom packaging suite. When changing materials, the personnel must exit the cleanroom and change gowns, masks and gloves before beginning the next subdivision process.
Some raw materials such as salts, buffers, amino acids and carbohydrates, have an intrinsic propensity to form clumps or cake in storage. Breaking up these clumps is an additional, time-consuming step that cleanroom personnel must complete to measure out the precise amounts needed for bioreactor processes.
All these procedures must be carefully documented according to cGMP standards. The personnel in cleanroom packaging suites need to be highly trained, since working in clean environments demands knowledge, discipline and motivation, as well as a thorough understanding of contamination risks among all personnel involved.
Managing the flow of materials through these cleanroom packaging suites is a constant challenge. Avantor’s J.T.Baker Direct Dispense packaging technology offers ways to help streamline cleanroom operations, reduce risks and eliminate the potential for production bottlenecks.
This packaging option provides single-use, pre-weighed and free-flowing product in specially designed, transparent polymer bags that are available to pack up to 100 kg of material. These bags dispense salts, buffers and other cell culture materials directly into their media or buffer preparation tanks, in the exact amounts specified for a given process (figure 1).
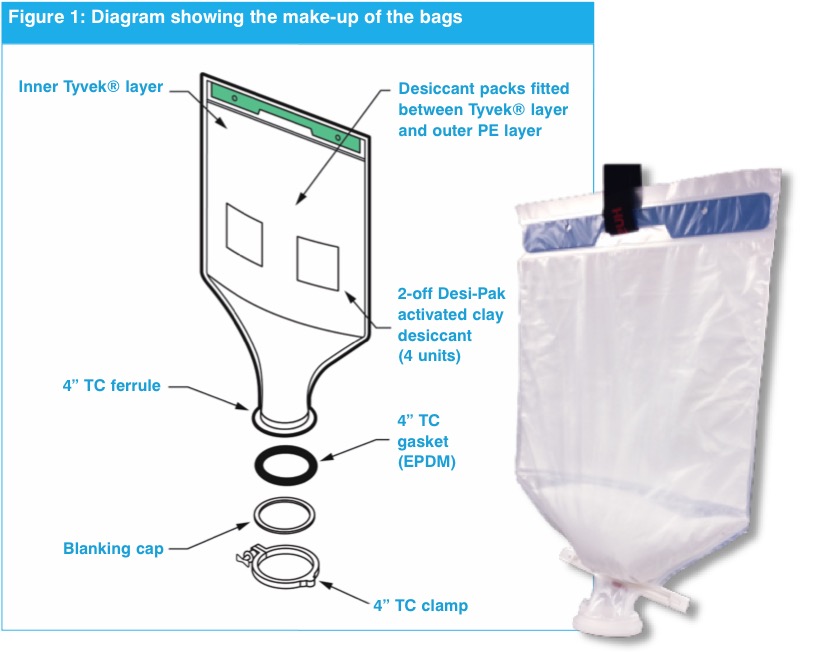

To help ensure a free-flowing dispensing system and eliminate clumping, some bags have outer and inner layers with special desiccant materials between the two layers. The inner layer uses a vapour permeable material; any moisture that develops within the bag passes through this material and is controlled by the desiccant, to maintain the correct moisture levels
Materials in Direct Dispense packaging do not need to be processed through cleanroom packaging suites. They can be received directly into inventory and then delivered to production as needed. While many products will still be supplied in bulk containers (requiring sampling upon receipt and then subdivision as needed), incorporating use of Direct Dispense packaging can “lighten the load” on cleanroom suites, enabling more flexibility in using these facilities.
The size, shape, sealing and seams of these bags are designed so that when they are inverted, they dispense virtually all the pre-weighed material into the bioreactor. An important consideration here is that pre-weighed and dispensing amounts are within a 1% tolerance of the amount of material required. In addition, Direct Dispense bag systems are compatible with non-destructive identity-testing tools, such as contact-free Raman spectroscopy. There is no need to open the bag and take a physical sample to verify the product; the closed bag can be scanned and verified upon delivery, saving multiple testing steps.

With new developments in raw material packaging, even after four weeks at 40°C and 90% relative humidity, the material in this 10 kg bag remains free-flowing
Time and cost savings
Expanding the use of Direct Dispense systems provides a flexible manufacturing technology designed to help biopharmaceutical manufacturers maximise the efficiency and utilisation of cleanroom packaging suites. Efficiencies from the use of these systems include:
Labour: eliminates the time and cost of personnel who need to weigh, subdivide and dispense materials from bulk containers.
Cross-contamination: the risk of cross contamination — by trace elements not properly removed from the cleanroom after a prior subdivision step, or contamination from cleanroom personnel failing to follow antiseptic procedures — is essentially eliminated.
Facilities: use of Direct Dispense systems can eliminate the need for dedicated raw material cleanroom prep areas, drum storage and handling equipment, and environmental (temperature and humidity) controls for those areas.
Testing/validating: use of Raman testing and tailgate samples greatly simplifies the testing identification step.
Quality: pre-weighed Direct Dispense systems eliminate the need to clean the weighing and dispensing area for another operation, thereby saving time and reducing cross-contamination risks.
Material stability and efficient use: reduced-clumping packaging design improves raw material yields by avoiding material non-conformities and inaccurate ingredient measurements from severely clumped materials.
