The requirements for CO2 incubators also include creating ideal growth conditions for patient samples and optimised shelving that ensures a high throughput per footprint.
Important considerations for cleanroom compatibility and cell therapy manufacturing
1) Cleaning and disinfection compatibility
2) Very low particulate emission and technologies to limit microbial contamination
3) Complete certificate and documentation package
4) Optimised space for large vessel types to achieve a high throughput
1) Cleaning and disinfection compatibility
For a CO2 incubator used in cell therapy development and production, a brushed 304 stainless-steel exterior is recommended. It is more resistant than a traditional painted steel exterior, for use with chemical disinfectants for manual cleaning and with vaporised hydrogen peroxide (VHP) procedures. Inside the incubator chamber, interior components that are electropolished stainless steel reduce microscopic structures that could provide areas for microbial growth. Ingress protection 54 (IP54)-rated exterior casing and a silicone-sealed touchscreen display protect the electronics, further supporting compatibility with common cleanroom protocols.
2) Low particulate emission and technologies to prevent from microbial contamination
Since a final cell-based product to be injected into a human patient is inherently limited in the amount and ways that it can be purified, particulate management remains a primary concern in the cell therapy manufacturing setting. Microbial contamination remains the number one cause of recalls for biological pharmaceuticals. But these are not the only microscopic contaminants of concern. Non-viable particulates were the reason for 22% of US FDA recalls of sterile injectables from 2008-2012[1] and the second leading cause of recalls from 2009 to 2019 (3). Tiny articles from fabrics, stainless steel, glass, plastics and more represent a variety of dangers for a patient, from an unintended immune response to a pulmonary embolism. And since the production equipment itself is responsible for an estimated 15% of particle generation in a cleanroom[2], equipment that helps to control particulate emissions is extremely advantageous[3].
A CO2 incubator that offers an active particle control system to control the emission of particulates in the air is proven to be highly suitable for use in an ISO Class 5 and Grade A/B cleanroom environment.
As your partner in production, a CO2 incubator should also offer proven solutions for contamination control and maximum time at set parameters so that cells express the proper characteristics and potency. An in-chamber circulating fan not only provides fast recovery from a door opening and optimised uniformity, but also the opportunity for in-chamber HEPA filtration. With a carefully designed air circulation system, recovery of all parameters in ten minutes following a thirty second door opening can be achieved. The same system driving HEPA filtration can reach ISO Class 5 conditions in five minutes or less, capturing particulates of any size. An on-demand dry heat sterilisation cycle should be tested by an independent third party laboratory, working according to the standards in the international pharmacopeias to reach a 12-log Sterilisation Assurance Level (SAL). These standards also require continuous air circulation during the entire cycle. Forty-eight point temperature mapping demonstrates that all areas reach and maintain the specified sterilisation temperature, to ensure there are no cold areas where microbes could survive and regrow.
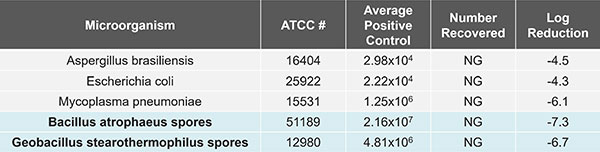
The table above shows results from a proven sterilisation, showing greater than six-log reduction of Bacillus atropheus spores, the specified biological indicator for dry heat sterilisation, and of Geobacillus stearopthermophilus spores, the specified biological indicator for autoclave sterilisation.
This cycle held at 180 ˚C for forty-five minutes. Doubling of this sterilisation time at 180 ˚C represents an “overkill” approach for a total 12-log SAL.[4]
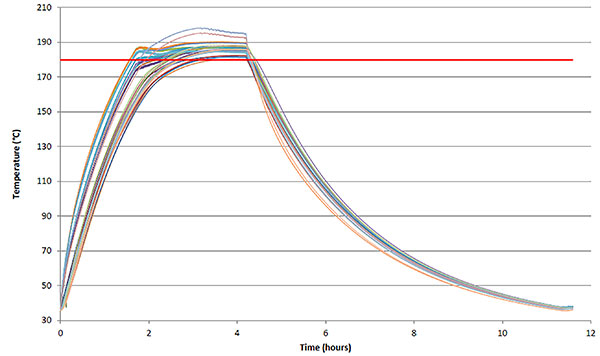
The figure above shows a forty-eight point temperature map during a CO2 incubator dry-heat sterilisation cycle during which all areas reach and hold at 180 ˚C or greater for at least ninety minutes.[4]
3) Certificates and documentation
A certified cleanroom-compatible CO2 incubator is one that has been tested according to ISO 14644-1 requirements and is proven to adhere to air quality limits within grade A/B cleanroom standards. In addition to testing the design, each unit should undergo complete end of line testing. To facilitate the audit process for on-site qualification, an equipment manufacturer should provide certificates and documentation to cover 1) product certificates, specifications and materials of composition 2) Factory Acceptance Test documentation and 3) clear guidance for cleaning, disinfection, routine maintenance and replacement of parts and accessories, as well as information on performance.
4) Optimised space for large vessel types to achieve a high throughput
With the right culture vessel and an environment that promotes proper growth, high expression of desired receptors, and optimum critical quality attributes (CQAs), CO2 incubators designed for cell therapy production can be part of an efficient scale-out program. Recognising the importance of maximising efficiency and valuable cleanroom space, recently available optimised shelving systems further maximise CO2 incubator chamber space to provide a high throughput per footprint.
Standard shelving systems are limited in terms of weight capacity and efficient use of incubator interior space. But by re-thinking shelving with designs specific to selected vessel types that better manage heavier loads, the capacity and handling can improve tremendously.
As one example, the standard shelving system for the Thermo Scientific™ Heracell™ Vios™ 250i CR CO2 Incubator holds four of Wilson Wolf G-Rex® 500M-CS bioreactors. With an optimised shelving system, the capacity can be increased to ten of the G-Rex® 500M-CS units, an increase of 150 %.

Since in a cleanroom production environment, most CO2 incubators are stacked, the new shelving provides a potential capacity of 20 G-Rex® 500M-CS per incubator footprint (as per example 25.1 in x 34.6 in). Each of Wilson Wolf’s G-Rex® 500M-CS can produce up to twenty billion cells in ten days. Regardless of whether the production goal is autologous or allogeneic immune cell therapy, this is an impressive output per footprint. The production volume combined with the cost-effectiveness of using CO2 incubators compared to standalone bioreactors such as rockers supports the attractiveness of CO2 incubators for cell therapy development and manufacturing. For these critical applications, it is important to carefully evaluate all features, ensuring proven performance.
1. Tawde, SA. (2015) Particulate matter in injectables: Main cause for recalls. Journal of Pharmacovigilance 03.2. Clarke D, Stanton J, Powers D, et al. (2016) Managing particulates in cell therapy: Guidance for best practice. Cytotherapy 18(9):1063-1076.
3. Thermo Scientific Smart Note: What is a certified compatible CO2 incubator design, and why is this an important consideration for any cell therapy or gene therapy process? (2021) Thermo Fisher Scientific COL34101 0321.
4. Thermo Scientific Steri-Run sterilization cycle proves total sterilization. (2015) Thermo Fisher Scientific ANCO2STERIRUN 0215.
