Wet contact time is one of the critical parameters in choosing a disinfectant (a chemical agent used for microbial reduction purposes, e.g. sporicide or sanitiser) for use in a classified area. Disinfectant contact time is commonly known as "wet contact time", "contact time," "dwell time," or "action time." This article emphasises the importance of this wet contact time for effective disinfection.
The appropriate wet contact time is determined during disinfectant qualification (coupon studies), by demonstrating an effective log10 reduction of microorganisms against a specified wet contact time. Therefore, the minimum contact time is the time needed for the disinfectant to achieve the necessary log reduction. Consequently, the facility surfaces need to remain wet with the disinfectant for the contact time that was qualified during the in vitro coupon studies.
Disinfectant chemical reactions need water as a solvent to allow the reaction to occur
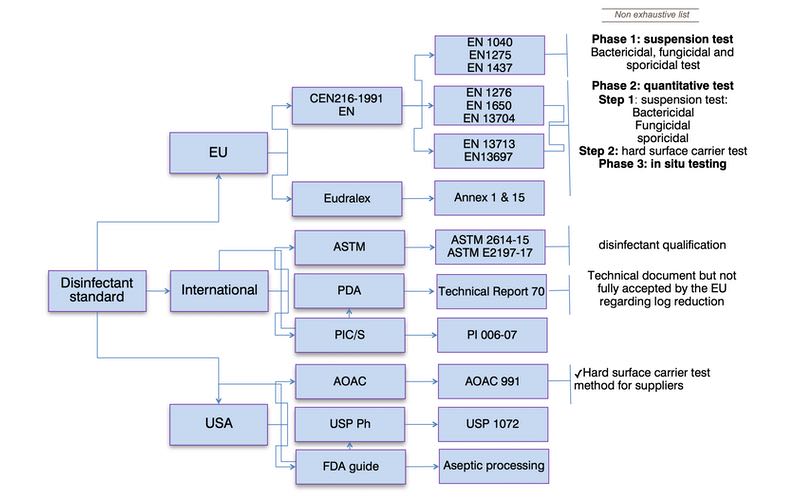
The regulatory guidance and industry technical documents require (bio)pharmaceutical manufacturers to qualify a disinfectant wet contact time (in vitro testing). Manufacturers are also required to validate the disinfection process by utilising the qualified disinfectant wet contact time in the real world (in situ testing) to demonstrate the effectiveness of the cleaning and disinfection programme as seen in figure 1.
Wet contact time
Disinfectant manufacturers must register their disinfectants based upon a specific wet contact time, microbial kill, and disinfectant concentration in compliance with the relevant authority's regulations. Pharmaceutical manufacturers must then demonstrate disinfectant efficacy on their cleanroom's surfaces.
Different surface coupons representative of surfaces in the cleanrooms are used to qualify the disinfectants. This qualification is based on the wet contact time, log reduction acceptance criteria, disinfectant concentration, water quality, surface types, and the microbial kill. The wet contact time during in vitro testing generally depends on:
- Microbial kill
- Disinfectant formulation
- Disinfectant concentration
- Cleanroom surface
The wet contact time and the inactivation of microorganisms in the cleanroom (real-world setting) generally depends on:
- Temperature and humidity in the cleanroom
- Air exchange in the cleanroom
- Application technique (e.g. vaporisation)
- Surface cleanliness
- Time for the disinfectant to dry
These parameters are generally not tested during the disinfectant qualification (in vitro). Therefore, it is required to demonstrate that these parameters do not affect the disinfectants' efficacy in the cleanroom.
Scientific and regulatory perspective
A study by West et al. evaluated the bactericidal activity of registered disinfectants after the disinfectant dried on the surface and found that there was no additional bactericidal efficacy after the surface dried. This is a finding that is agreed with many regulatory organisations.
Parenteral Drug Association Technical Report 70 (PDA TR 70) Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities, defines contact time as, "The minimum amount of time that a sanitizer, disinfectant, or sporicide must be left in complete (wet) contact with the surface to be treated in order to be effective". PDA TR 70 also states, "Provided the appropriate chemical agent is used, the key to disinfection in the cleanroom is to ensure that the surface remains wetted for a sufficient period of time for the chemical to accomplish its mode of activity". In addition PDA TR 70 directly connects the wet contact time qualified during the in vitro coupon studies with the wet contact time in practical application in an aseptic manufacturing facility, through the following statement: "Curtain surfaces should be sprayed or wiped with an efficacious disinfectant or sporicide and allowed to remain wetted for the contact time validated in the antimicrobial effectiveness studies."
It is not relevant to consider dry contact time to be equivalent to the wet contact time
Furthermore, the US Center for Disease Control (CDC) defines contact time as, "Time a disinfectant is in direct contact with the surface or item to be disinfected. For surface disinfection, this period is framed by the application to the surface until complete drying has occurred"
As an example, a 2018 US FDA Form 483 observation stated: "Specifically, your firm did not have adequate contact time for the (b) (4) ((b)(4) solution) or the (b) (4) for use as a sporicidal. Your technician sprays the solution onto a sterile wipe and then wipes down the surface of the ISO 5 hood, amounting to a contact time (surface being wet) of less than (b)(4).", specifying that contact time is the time that the surface remains wet. Additionally, a 2019 FDA Form 483 observation stated, "...inadequate amount of cleaning/sanitizing agent ((D) (4)) was used to ensure an appropriate wet contact time on floors". Consequently, when the disinfectant dries on the surface before the qualified wet contact time is achieved, a reapplication of the disinfectant should be performed, to ensure that appropriate microbial control is maintained and that regulatory expectations are met.
The appropriate wet contact time is determined during disinfectant qualification (coupon studies)
For these reasons, it is not relevant to consider dry contact time to be equivalent to the wet contact time. If the validated wet contact time does not need to be achieved in practical application, this stance implies that the contact time is potentially indefinite (or at best, unknown). This stance also implies that the contact time continues until a level of degradation of active ingredients occurs to a minimum effective concentration, or until the disinfectant is rinsed from the surface. If a minimum wet contact time is not achieved consistently, then the pharmaceutical manufacturer does not have sufficient control of a critical process, which violates cGMP principles and increases the risk to the medicinal product and, subsequently, to patients.
Do not forget
Disinfectant chemical reactions need water as a solvent to allow the reaction to occur, which demonstrates why the wet contact time is paramount to ensuring that microorganisms are inactivated. It is clear from a scientific, regulatory expectation, and from a critical process control perspective, that achieving a minimum wet contact time is essential for maintaining an effective disinfection process and preventing microbial contamination proactively.
The validated wet contact time allows for demonstrated, effective inactivation of microorganisms in the cleanroom, irrespective of cleanroom parameters or application techniques, that may affect the duration of time the surface stays wet on the cleanroom surface. Disinfectant activity is halted or significantly reduced when insufficient liquid is present, and it is not possible to adequately control the disinfection process, without a minimum wet contact time. Consequently, if a minimum wet contact time is not achieved, the disinfection process effectiveness is not assured, and the manufacturer's risk is increased.

