During the last decade, the infection control sector has been facing new challenges. These include demographic growth leading to overcrowded hospitals; antibiotics misuse, increasing pathogen resistance; new pathogenic threats, such as Prion-associated diseases; and sophisticated medical devices and instruments demanding new technologies for reprocessing methods and adequate monitoring indicators.
The continued technological advances and market needs have greatly changed infection control concepts. For this reason, medical device manufacturers, as well as central sterile services department (CSSD) had to quickly adapt to the modern demands. The same goes for cleaning, disinfection, sterilisation and associated monitoring protocols, not to mention regulations and standards.
Between the different hospital departments involved in infection control, the CSSD is, unquestionably, the most essential in pathogen transmission avoidance. The CSSD is responsible for providing sterile material to doctors, nurses and surgeons and, in this task, cleaning, disinfection and sterilisation monitoring play a key role.
Physical, chemical and biological monitoring are the three crucial tools inside any CSSD. Physical controls correspond to specific sensors built in equipment and not the focus of this article. Moreover, it is not best practice to rely solely on physical measures from the equipment itself; any malfunction leads to risking an undetected cycle failure.
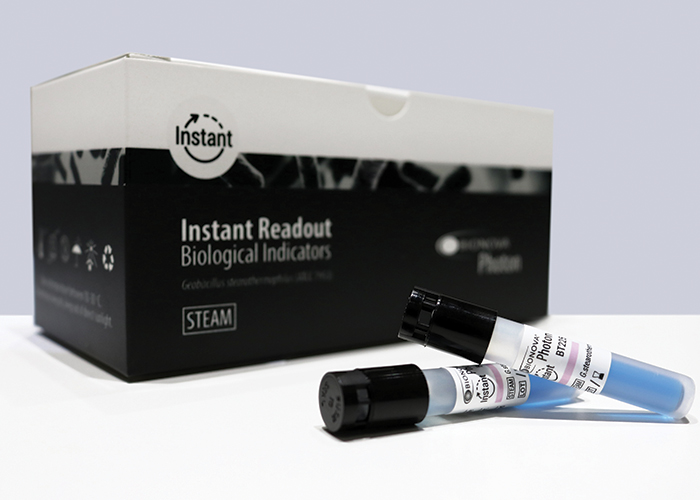
The PHOTON Instant BI has been specially designed for steam sterilisation processes in vacuum-assisted autoclaves
For the past 50 years, chemical indicators (CIs) have become a leading method. Thanks to a recognisable colour change CIs are easy to interpret, and with the emergence of type 5 and 6 indicators, they provide instant and precise results. It is undeniable that CIs served their purpose. However, CIs are limited to monitoring process parameters, and even if they can simulate or emulate certain conditions, they will never replace the biological indicator (BI), which contain spores: the most resistant form of life.
It is irrefutable that BIs represent the best challenge for sterility assurance because they are the only real and direct evidence of microbiological death. This is why all BI manufacturing companies are investing and developing self-contained BIs with faster incubation times and minimal user intervention, leading to the race towards the fastest and easier-to-use BI in the market.
Faster readouts
Forty years ago, spore strips were the BI of choice, mainly because it was the only microbiological available technology. This type of BI involves steps that require not only specific microbiological skills by the operator but also special sterile, not to mention expensive, facilities and very long incubation times; seven days or more.
These were not the only disadvantages of the system. There was also a constant risk of cross-contamination during the strip manipulation, thus giving false positive results.
More recently, self-contained BIs (SCBIs) have come to light with synthetic mediums that allowed shorter incubation times; between 24-48 hours. This BI setup, however, also added an extra challenge to the sterilising agent penetration, thus acting as a process challenge device (PCD) itself. Despite this system could solve the cross-contamination risk, it was not sufficient to release the load with enough speed and to fulfil the demands of faster instrument turnover.
Harnessing biotech and microbiology breakthrough and exceptional investment, Terragene has been able to develop a complete line of BIs with fluorescence-based readout, compatible for steam, hydrogen peroxide, formaldehyde and ethylene oxide sterilisation processes. The technology allows the development of BIs with incubation times as short as 20 minutes with a prediction higher than 97%.
New BI technology has made necessary the development of a suitable reading system: the Bionova Automatic Reading Incubators. With it, the user simply takes the BI, activates and put it in the incubator with the appropriate programme. Once the programme is finished, the result is given automatically without interpretation or intervention by the operator.
Attending Medica 2018 in Düsseldorf, Terragene celebrated the launch of the first instant biological indicator featuring fluorescence emission and readout: the PHOTON Instant BI. It has been specially designed for steam sterilisation processes in vacuum-assisted autoclaves.

New technology is compatible with Bionova auto-readers
By using any of the available Bionova auto-readers, the user will be able to confirm the effectiveness of the sterilisation cycle in only a few seconds! Photon BI is undeniably a new milestone in infection control, giving the CSSD unprecedented speed for releasing the load with highly sterility assurance. The product is compatible with the range of Bionova auto-readers.
The research and development behind this project was also aimed to elaborating an exclusive software capable of saving the BI results automatically, linking the information to the sterilisation cycle, operator, steriliser, BI batch details, etc. With the Bionova traceability software, the user can monitor the historical performance of every steriliser inside the CSSD independently and to detect malfunction and/or operator error.
Of course, all these improvements must be accompanied by new regulations and recommendations to emphasise the importance of load release based on BI routine use, quality control and traceability of CSSD’s processes.
Great effort has been delivered towards simplification in BI daily use and its traceability, giving hospitals the chance to improve their infection control protocols without unnecessary expenses. This way, CSSD managers can focus their efforts on training staff or better technology for their processes, rather than spending time with out-of-date and difficult to achieve practices.
CSSD staff can now release the load by using BIs with short/instant reading times, where spores directly challenge the process and an automatic electronic system informs the results with unprecedented reliability and sensibility.
N.B. This article is featured in the December 2018 issue of Cleanroom Technology. The digital edition is available online.
