The pharmaceutical industry is constantly evolving in response to health threats. For example, the COVID-19 pandemic has caused a substantial investment in vaccine production over the last few years, leading to new developments like mRNA. Oncology has been another area of significant progress in recent years. But, these unprecedented advancements entail various risks for workers in the pharmaceutical industry. In this article, Steve Marnach, EMEA Training manager & Specialist Critical Environments at DuPont, discusses emerging threats, new legislation, and selecting personal protective equipment (PPE) like cleanroom garments.
Health and contamination risks in pharmaceutical applications
Many pharmaceutical applications use hazardous substances that can put workers at risk. These substances could be chemical or biological and come in the form of gases, liquids, and solids.
According to CLP regulations, there are three main categories of dangerous substances - physical hazards, health hazards, and environmental hazards. PPE plays a substantial role in protecting pharmaceutical workers from health hazards.
Exposure can lead to serious health effects, even at low concentrations
High potency active pharmaceutical ingredients (HPAPI) are among the most dangerous substances found in the industry. Exposure can lead to serious health effects, even at low concentrations. Symptoms range from allergies and skin diseases to cancers and reproductive problems. As the demand for HPAPI is growing globally, protecting workers in pharmaceutical manufacturing is vitally important.
Selecting appropriate PPE is critical to safeguarding workers' health and safety. It can also reduce the risk of contamination in pharmaceutical applications.
Reports indicate that 75% of all contamination inside cleanroom environments comes from workers and their clothing. The consequences of this contamination can be severe, including a shutdown for cleaning and decontamination or even a product recall.
Legislation and guidelines for pharmaceutical PPE
In Europe, REACH (Registration, Evaluation, Authorisation, and Restriction of Chemicals) legislation regulates dangerous chemicals. An onus is placed on organisations to identify and manage the risks associated with the substances they import, use, or manufacture.
Other regulations specifically aim to protect workers from carcinogens, mutagens, and reprotoxic (CMR) substances. For example, Directive 2004/37/EC mandates using risk assessments, preventive measures, training, and PPE to protect workers against CMR products. The EU Council approved an amendment to this directive in March 2022, which introduced exposure limits for acrylonitrile and nickel compounds. The new directive also lowered Benzene limits, increased protection against reprotoxic substances, and mandated training on handling hazardous medicinal products safely.
Legislation states that PPE "should be used when all other measures are inadequate to control exposure.”
Regulations covering contamination are also evolving. For example, the final version of the EU's Good Manufacturing Practice (GMP) Annex 1 guideline, covering the current regulatory and technological developments in the manufacture of sterile medicinal products was published on 25 August 2022. As a result, pharmaceutical manufacturers will face additional requirements to proactively identify and control potential risks to quality associated with cleanroom garment systems. This is in line with QRM (Quality Risk Management) principles.
According to COSHH (Control of Substances Hazardous to Health) legislation, equipment like protective clothing is a worker's last line of defence. This legislation states that PPE "should be used when all other measures are inadequate to control exposure.”
PPE for vaccine manufacturing
The vaccine market has grown significantly during the COVID-19 pandemic. This expansion is set to continue, with the WHO targeting 70% COVID-19 immunisation in 2022. Messenger RNA (mRNA) vaccines have been especially effective in combatting COVID-19. These vaccines are quicker and easier to produce as they do not use cell cultures with high-level biosafety containment. Instead, they use chemical processes with hazardous active ingredients and organic reagents.
PPE plays a significant role in protecting workers in mRNA manufacturing environments. The garments should be CE-certified as category III, protecting against severe or fatal risks. However, several different types within that category depend on the application and risk level. The figure below summarises these types and the protection offered. For mRNA applications, protection against aqueous liquids and liquid aerosols is a common requirement. Materials made from high-density polyethylene filaments form a tight homogeneous fabric that protects wearers against particles down to 1 micron in size.
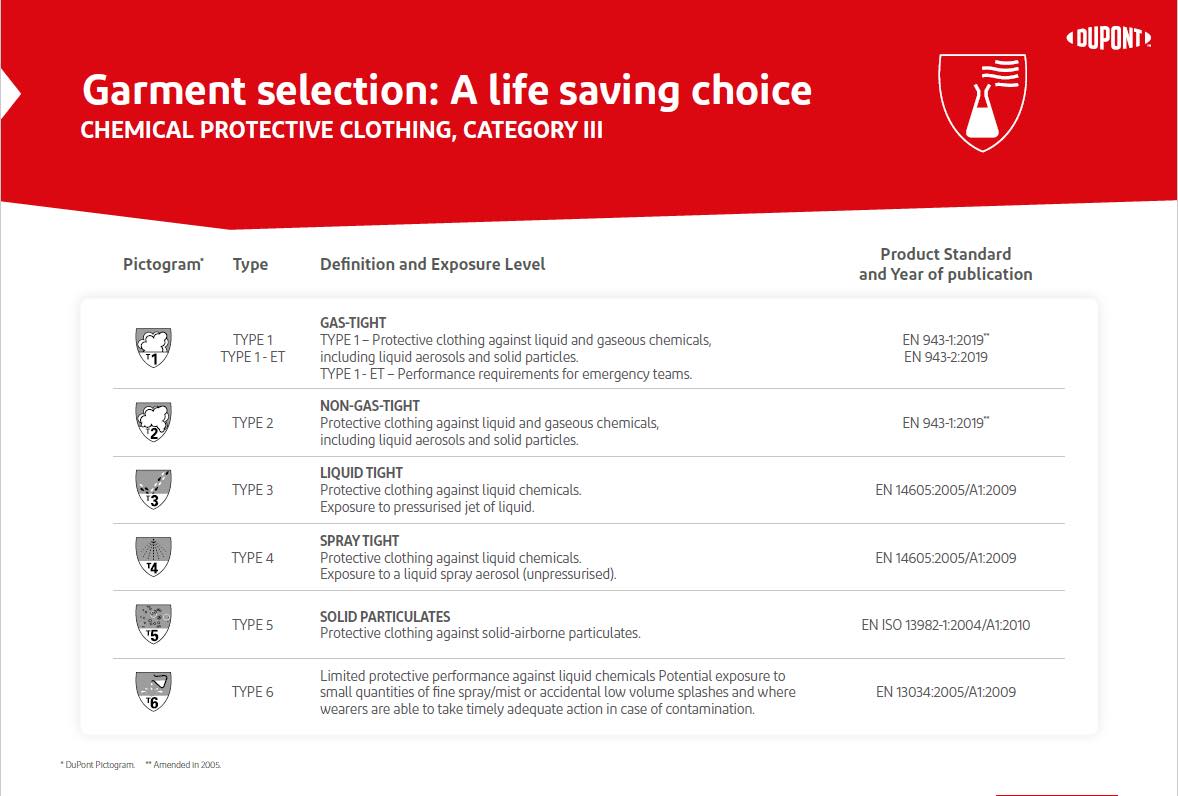
Cleanroom PPE must also prevent contamination. Garments must be suitable for either an A/B or C/D grading, depending on the cleanroom type. They must also be abrasion and tear-resistant. There are three types of tests to assess and validate the effectiveness of cleanroom PPE:
- The Helmke drum test measures particles generated by the garment
- The particle filtration efficiency (PFE) test and the bacterial filtration efficiency (BFE) test assess how well the garment prevents particles shed by the wearer from escaping
- The body box test assesses particle shedding from the garment and the wearer and the garment's PFE and BFE
Comfort is also an essential consideration for PPE because uncomfortable garments can cause sweating, increasing particle shedding. Lower comfort also leads to fatigue and injuries.
PPE for oncology drug production and preparation
The oncology market is also experiencing rapid growth, with 64 new active substances released in the last five years. Oncology drugs are becoming more effective but also more hazardous to make.
Cytostatic compounds, carcinogenic, mutagenic, and reprotoxic, are commonly used in oncology drug manufacture. Exposure to cytostatic dust, liquids, or aerosol formation risks workers. Another cancer treatment called antibody-drug conjugates (ADCs) uses powdered cytotoxic reagents. Healthcare workers in hospital pharmacies are particularly at risk of exposure from skin contact, inhalation, or needle stick injuries.
Symptoms of exposure range from contact dermatitis and allergic reactions to miscarriage or foetal malformations in pregnant women.
PPE for the oncology drug production environment should be CE certified as category III. Workers may also require a high level of protection (Type 4-B, 5-B, and 6-B) with the comfort of a non-woven suit. Chemical-resistant socks ensure that workers' feet are fully sealed and protected while stitched and over-taped seams reduce the risk of particle shedding. Tunnel elastics for cuffs, ankles, and face, along with a tight fit around the respirator, reduce the risk of contamination.
Workers performing maintenance or cleaning tasks require higher levels of protection due to their risk of exposure to dangerous compounds.
Conclusion
Workers in the pharmaceutical environment need protection from the hazards they face, and PPE is an essential last line of defence. PPE also prevents contamination in cleanrooms which is vital for the safety of drugs entering the marketplace.
DuPont offers a wide range of PPE with CE certification as category III. These garments protect workers against severe or fatal risks. Tyvek filaments are thermally bonded into a tight homogeneous fabric that protects wearers against particles larger than 1 micron. These garments are lightweight, soft, and breathable, thus ensuring optimal comfort for workers.

