What does data mean to you? Look at the data set in Table 1 and imagine those numbers are from the particle count you have on any location from your particle monitoring system.
Think about what this data alone means to you: is this data valuable as it is now? Are there any other contributors you need to analyse and make it useful for your cleanroom and production?
Most end-users are collecting particle count data from their state-of-art online particle monitoring systems. However, due to lack of data analysis and assessment, either they overestimate conditions and take unnecessary actions that create a chain of failures, or neglect major issues and assume that they are safe, just because they have a monitoring system in place.
If you look at the table again, these numbers can be anything. Without proper records attached to this numbers, such as units, time, location, cleanroom class, occupancy, location status (during live production or passive conditions) or data tags, such as operators alarm acknowledgement notes, these numbers don’t mean anything to anyone. These numbers, in tandem, could be authors mobile number, too.
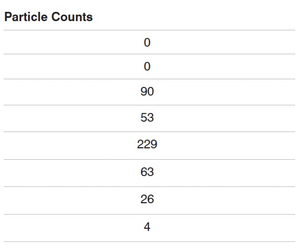
Table 1: Data set from particle counter
Data analysis and assessment of warning and alarm conditions, as per predefined limits, is also critical. Let’s complete all missing parameters and analyse data again, as shown in Table 2.
Now we have all we need to analyse and interpret this alarm condition. First of all, we’ve got much valuable information about conditions during this alarm:
- This alarm happened at a turntable from aseptic vial production
- It was during production
- Alarm status caused by operators’ intervention, not due to routine production conditions
- Alarm ended right after this intervention and particle counts back to its normal conditions
We know that this kind of alarm with known sources are easy to manage. The only thing, for now, is monitoring the vial, so it does not become a route cause, and operator interventions are not required too often.
Also, studying potential failure modes and testing them during media fill is critical to determine alarms and their results to product quality and patient health.
Try to limit your operators’ alarm acknowledgement sentences. A good practice is to limit them with predefined texts that are tested scenarios during media fill. Other alarm reasons will also help you to investigate and address problems other than sweeping them under the rug.
If the alarm is not addressed and repeats itself frequently, that is something that you should investigate. This is not to meet guidelines and criteria but to avoid potential risks for the patient and your product, which could be fatal. Having an online monitoring system, collecting data, printing them for batch records and acknowledging alarms are not our ultimate target.
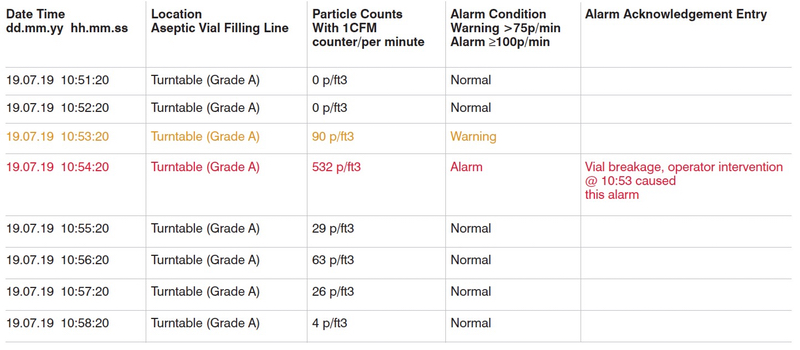
Table 2: Cleanroom monitoring data
Trend Reports
The same approach is valid for trend reports. Trends are not just about sampling the same location data at a specific period, e.g. year after year. It doesn’t prove anything to anyone if you are only recording data the same day in two different years. Also, it will not help you to identify system conditions and year on year status. However, if you apply trends to the same location for the same period of each batch and get the data with statistics, such as average, mean and standard deviation, this can help you to analyse data and to see the difference.
Searching peak repeats will help you to understand whether they are just irregular peaks or happen right at the same time at the same event. For example, getting peaks every time at a stopper bowl location right after adding more stopper indicates poor design and needs attention.
Searching peak repeats will help you understand whether alarms are just irregular peaks or are simultaneous with other events
Peak counts at a turntable right after opening a depyronisation tunnel shouldn’t be a repeating alarm condition. As stated in GMP Annex 1: “Consecutive or regular counting of low levels is an indicator of a possible contamination event and should be investigated. Such events may indicate early failure of the HVAC system, filling equipment failure or may also be diagnostic of poor practices during machine set-up and routine operation.”
The standard deviation can help you to identify the trend of your average data. If your standard deviation increases event by event, this may help you to diagnose the early failure of your entire design.
Data, tables and graphs
Like in trends, having all location data printed for your batch records may not help you. Over 100 pages of data at your batch records may become a stack of papers in the long run. The entire software platform must be validated, and all data securely captured to your database. Having alarm event reports and audit trial printouts will assist you in evaluating current condition, production health, alarm conditions and their acknowledgements. It will tell you whether all alarms and their acknowledgements are based on what you study during media fill or whethere there is something else that you need to take action on.
System maintenance
Most online particle counters need service during the calibration period. Without having major failure and service level agreement (SLA), most users in the industry keep units on their premises for 12 months. If your online particle counter has lack of self-diagnostics, a disaster might be waiting for you.
During calibration, some particle counters can be diagnosed as “deaf”. This means sensitive components, such as the photodiode laser or photodetector, stopped functioning somewhere between the last calibration to date. The device has nothing to do; zero is all it reads. But, what happened in reality? Nobody knows it. Significant compliance and major data integrity issues are waiting for you. So, self-diagnostics should be the first thing you check while investing for particle monitoring systems.
During calibration, some particle counters can be diagnosed as “deaf” when their sensitive components stop functioning
What is self-diagnostics? Simply, if your particle counter can talk to you about the current status, such as laser, background voltage, photodiode health, flow status, etc., this means you don’t need to worry about the health of your sensor. You will know that if anything happens, you will receive a message to your software telling you: “laser background voltage: bad”. Then you can take immediate action that will help your compliance and data integrity. If your system has no such an option, make sure you have routine testing for conditions. Talk to your supplier for more information and test plans that you can integrate into your maintenance SOP.
Predictive maintenance
Particle monitoring systems with a self-diagnostic feature are easy to maintain. There are a couple of issues that you should be aware of, including those relevant to the isokinetic probe, the monitoring period, and cleaning the unit.
- Isokinetic probe caps should be off after the batch cycle. Caps are protecting your particle counters from any spill or unnecessary particle exposure during maintenance of your cleanroom. Note that many models of particle counter can be cleaned when liquid penetration occur, but will definitely lower your product lifetime.
- Do not run your online monitoring system 24/7. Online doesn’t mean day and night. As per GMP, online means that “particle monitoring should be undertaken for the full duration of critical processing, including equipment assembly”. In general, it means starting before production to see if conditions are good to go, during the full production period and after production, including equipment disassembly. Such monitoring will also affect the lifetime of your pump and overall particle counter.
Another downside of the 24/7 approach is that you will never know midnight particle peaks without any reason, but you should acknowledge them and write it. Even GMP states: “The occasional indication of ≥5.0 m particle counts may be false counts due to electronic noise, stray light, coincidence, etc.”
So, stick to plan, use your online monitoring system during production with capturing before (preparation, assembly) and after sessions (closing, disassembly).
Do not run your online monitoring systems 24/7, it will affect the lifetime of your pump and overall particle counter
Clean your sample probe gently with IPA. Even online particle counters have special particle sampling tubes, and sometimes particles are accumulating at the inner surface of sample tubes. This particle penetration can be due to the size of particles, particle characteristics or even humidity changes inside your cleanroom.
Sometimes end-users are applying high-pressure clean gases to clean them or using ultrasonic cleaners. Both methods, or any other similar to these, should not be used. The reason for this is simple. If you try to remove them by mechanical forces, this will cause wear and tear in your sample tube. Just take your sample tube, put some IPA inside, make a U shape and move your hands up and down gently. After several movements of IPA from one end to another, remove IPA and let sampling tube dry on its own.
Service Level Agreement is important
SLA is a commitment between you and your supplier. This agreement can cover services, calibration, remote support and special needs that will reduce your risk.
No matter how advanced your online particle monitoring system is, an SLA is essential. The agreement will lower your risk and ensure that your system is up and running properly.
Each supplier offers a wide range of SLA’s with a different level of coverage, from remote support to express onsite support within a limited time. Contact your online monitoring system supplier to learn more about SLA options and coverage, and make sure you customise this SLA coverage based on your needs.
N.B. This article is featured in the December 2019 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.





