Disinfection section, 4.34 Annex 1 states that "…The disinfection process should be validated. Validation studies should demonstrate the suitability and effectiveness of disinfectants in the specific manner in which they are used and on the type of surface material, or representative material if justified, and should support the in-use expiry periods of prepared solutions."
Annex 1, which guides the manufacture of sterile medicinal products, doesn’t make any specific reference to the disinfectant efficacy test standards which should be used
This phrase is more specific than the previous version of the annex, with a clear expectation that disinfectants must be validated not only in the specific way in which they are used, usually in a cleanroom by wiping or mopping but also on the different types of surfaces found in an end user’s facility.
Disinfectant efficacy validation
Annex 1, which guides the manufacture of sterile medicinal products, doesn’t make any specific reference to the disinfectant efficacy test standards which should be used. There is no mention of the pass criteria that needs to be achieved for an individual disinfectant.
There are differences between EU and US testing methodologies
The decision on level of microbial kill will be based on room grade, routine environmental monitoring, risk assessment and will form part of the Contamination Control Strategy (CCS) for the facility. It is the responsibly of the end user to decide on the level of log reduction required and this could be different for different grades of room.
The standard EN test methods have different log reduction pass criteria, for bacteria, yeasts, fungi, bacterial spores and viruses, and also different pass criteria dependant on whether the test is in suspension, on a surface or including mechanical action. However, the EN tests are not specific to life science cleanrooms.
Disinfection efficacy tests
Qualification of a disinfectant is demonstrated through laboratory performance testing to show that the disinfectant is capable of reducing the microbial bioburden found. There are various national and international efficacy testing standards available to compare and qualify disinfectants. It is worth noting that there are differences between EU and US testing methodologies, the various standards use different test methods, pass criteria, and standard organisms.
Contec wanted to carry out its own extended surface testing
It is also important to note that most of the standards and methods available are NOT written specifically for cleanroom disinfectant qualification. Europe has a three-phase evaluation process for disinfectant testing that is defined by CEN group 216 and summarised in EN14485:2022 Chemical disinfectants and antiseptics – Application of European Standards for chemical disinfectants and antiseptics.
European efficacy tests
BS EN 14885:2022 is the “Standard of standards” and it details all of the European Standards available to which products have to conform to claim microbial activity. It is applicable to products to be used in the area of human medicine, veterinary areas, and in food, industrial, domestic and institutional areas. It is continually updated to reflect the current version of each standard developed by CEN/TC216, so is a really useful place to start to find the relevant and current standards needed for a disinfectant qualification programme.
The EN efficacy tests follow a tiered approach. Phase 1 tests are quantitative suspension tests which are simply used to establish that a product under development has bacterial, fungicidal, yeasticidal or sporicidal activity without regard to specific areas of application. These are not appropriate for use as part of a facility's disinfectant qualification programme.
There are two EN surface test methods for testing a disinfectant without mechanical action.
Phase 2 tests are broken down into two steps: Phase 2 - Step 1 tests are quantitative suspension tests to establish that a product has bacterial, fungicidal, yeasticidal, mycobactericidal, tuberculocidal, sporicidal or virucidal activity under simulated practical conditions appropriate to its intended use. As a suspension test, these tests also don’t meet the Annex 1 requirement of validating a disinfectant in the manner in which it is to be used, ie application onto a hard surface.
Phase 2 - Step 2 tests are quantitative tests to establish that a product has bactericidal, fungicidal, yeasticidal, mycobactericidal, tuberculocidal, sporicidal or virucidal activity when dried onto an inanimate surface under simulated practical conditions.
These tests are suitable for use as part of the disinfectant qualification of a cleanroom disinfectant. Tests must be carried out under the minimum / obligatory conditions if a claim against that standard is to be made, usually by a disinfectant manufacturer. But an end user can use additional test organisms or modify the temperature, contact times, interfering substances, and amount of starting inoculum. Remembering these are not standards specifically designed for the very low levels of contamination found in cleanrooms.
EN surface test without mechanical action
There are two EN surface test methods for testing a disinfectant without mechanical action.
EN13697:2015+A1:2019 has a pass criteria of a log 4 reduction for vegetative bacteria and a log 3 reduction for fungi and moulds. EN16777:2018 has a log 4 pass criteria for viruses. There is no Phase 2 Step 2 test for bacterial spores. So many people use a modified EN 13697 test method. For bacteria, fungi and viruses, the pass criteria for a surface test is one log reduction less than is required to pass a suspension test so a universally accepted pass criteria for testing spores on a surface is a log 2 reduction.
EN16615 is a quantitative test method for the evaluation of bactericidal and yeasticidal activity on non-porous surfaces with mechanical action employing wipes in the medical area (4- field test)
USP <1072> also suggests a log 2 reduction in spores is an acceptable pass criteria. The standard calls for the use of an interfering substance, even in the clean conditions of the test, 0.3% interfering substance is required. It is becoming more accepted that as the surfaces in cleanrooms have been precleaned that Water for Infection (WFI) can be used as the interfering substance.
EN surface test with mechanical action
Another phase 2, step 2 test is EN 16615:2015. This even more closely mimics how a product is actually used in a cleanroom as it includes mechanical action.
EN16615 is a quantitative test method for the evaluation of bactericidal and yeasticidal activity on non-porous surfaces with mechanical action employing wipes in the medical area (4- field test). It can be used for both wipes and mops, either presaturated or saturated at point of use.
As with EN13697, there is the option for clean and dirty conditions, and the interfering substance in the clean condition could be WFI
As with EN13697, there is the option for clean and dirty conditions, and the interfering substance in the clean condition could be WFI. The test is carried out with the wipe wrapped around a granite block weighing 2.3 – 2.5kg to standardise the wiping procedure and to simulate the average pressure when wiping. It has a slightly different pass criteria to the other test methods as two parts to the test need to be passed.
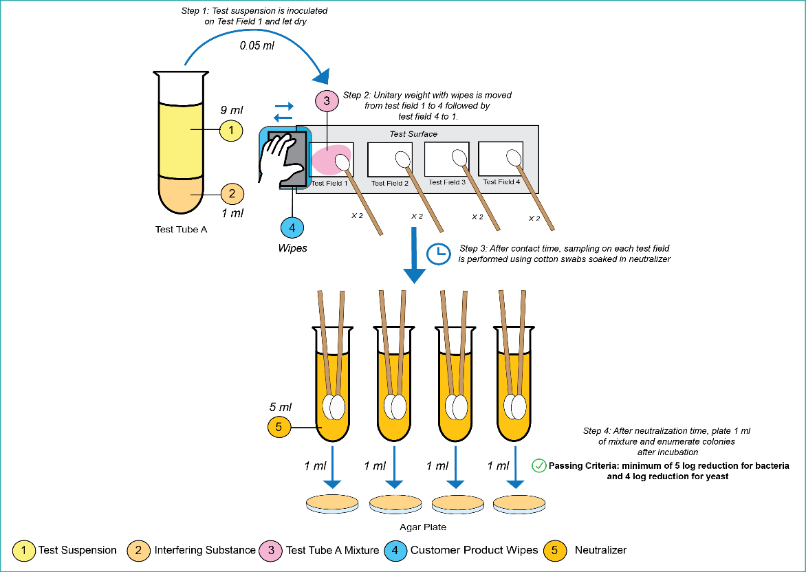
Figure 1: EN16615 test method
On test field 1, a reduction of log 5 in bacteria and a log 4 reduction in yeast is required to pass the test. And then additionally there must be an average of 50 cfu or less on test fields 2 to 4 for each organism (<50 cfu/25 cm2). This test can also be modified for fungal and bacterial spores with a log 1 reduction from the pass criteria for yeasts, so a 3-log reduction is often used as the pass criteria.
Choice of surfaces for testing
Disinfectant manufacturers usually carry out EN13697 and EN16615 surface testing on 316 stainless steel. However, this doesn’t mean that the disinfectant will work the same on all cleanroom surfaces. Many factors can affect the way microbes adhere to a surface. Surface roughness, surface charge, hydrophobicity / hydrophilicity, adsorption of organic molecules onto the surface, grade of material and surface damage or corrosion can all affect the level of log reduction achieved by a disinfectant.
Annex 1 now acknowledges this “Validation studies should demonstrate the suitability and effectiveness of disinfectants in the specific manner in which they are used and on the type of surface material, or representative material if justified...”
The justification should be provided to regulators if not all surfaces are tested. A matrix approach can be used including the factors that affect adhesion, surface roughness, porosity, etc
A surface risk assessment should be performed to indicate the “worst case” surfaces in the facility. Start by listing all the different surface types within your facility, from the cleanroom fabric to the critical equipment materials. Each surface should then be assessed for the impact they may have on the efficacy of the disinfectant. It is possible to score each material creating a risk score, and the materials with the highest risk should be selected for efficacy testing.
The justification should be provided to regulators if not all surfaces are tested. A matrix approach can be used including the factors that affect adhesion, surface roughness, porosity, etc. Also, consider the potential for chemical interaction at the surface of a material, the prevalence of the material within the rooms, contamination risk (horizontal versus vertical), and the proximity to the product.
Contec surface test work
We wanted to carry out our own extended surface testing for a variety of reasons.
- Was this new request in Annex 1 reasonable - Do we see a difference in efficacy on common surfaces for a sporicide and a broad-spectrum disinfectant
- To support customers who could maybe reduce their gap analysis if we could provide more efficacy surface test work than just 316 stainless steel
- To support the validation work for our own cleanrooms
We decided to conduct a study on 10 common cleanroom surfaces and 15 common cleanroom organisms. The choice of surface was influenced by materials in our own cleanrooms, being representative of the main surface types commonly found in cleanrooms and materials that were available at the test lab. We used EN13697 surface test without mechanical action, as this is the most stringent of the surface tests and it also meant we didn’t add in another variable of the type of wipe used.
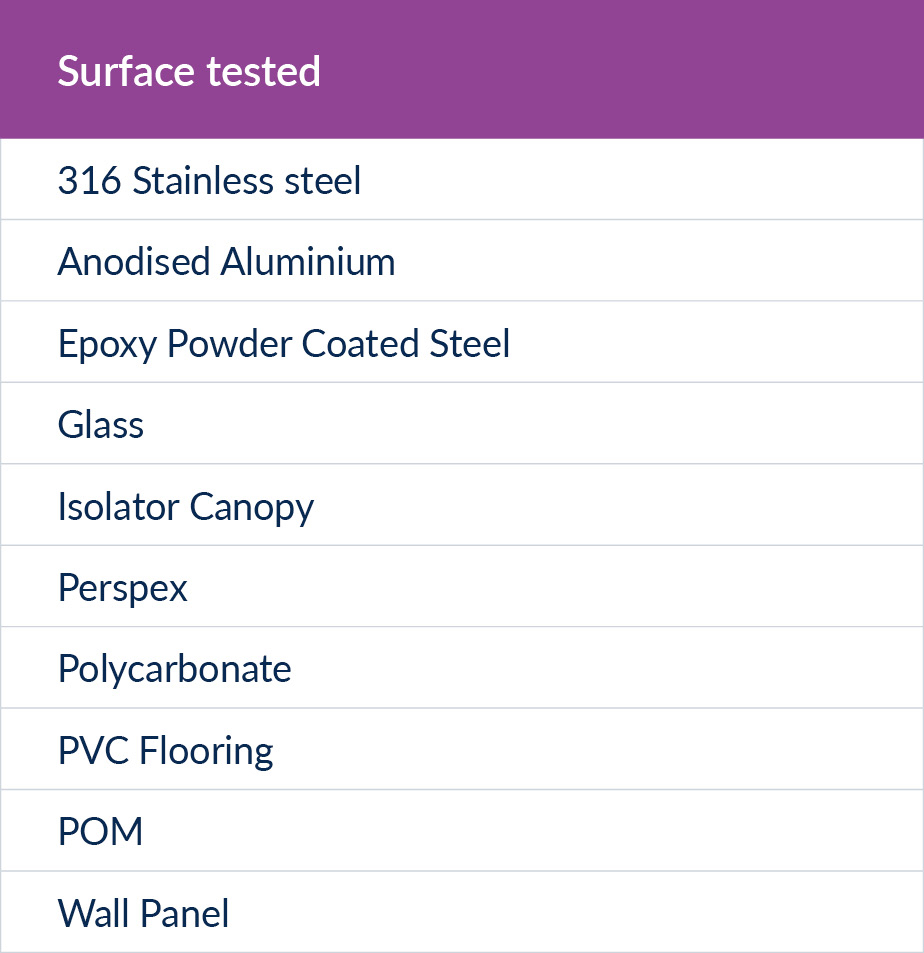
Figure 2: Surfaces tested
We wanted to carry out the work on more organisms than we saw routinely in our own cleanroom rooms so spent time analysing published work on the most common isolates found in cleanrooms European wide. We also asked for the most common house isolates submitted to two different disinfectant test labs and an identification test lab. We also chose to use isolates rather than ATCC strains.
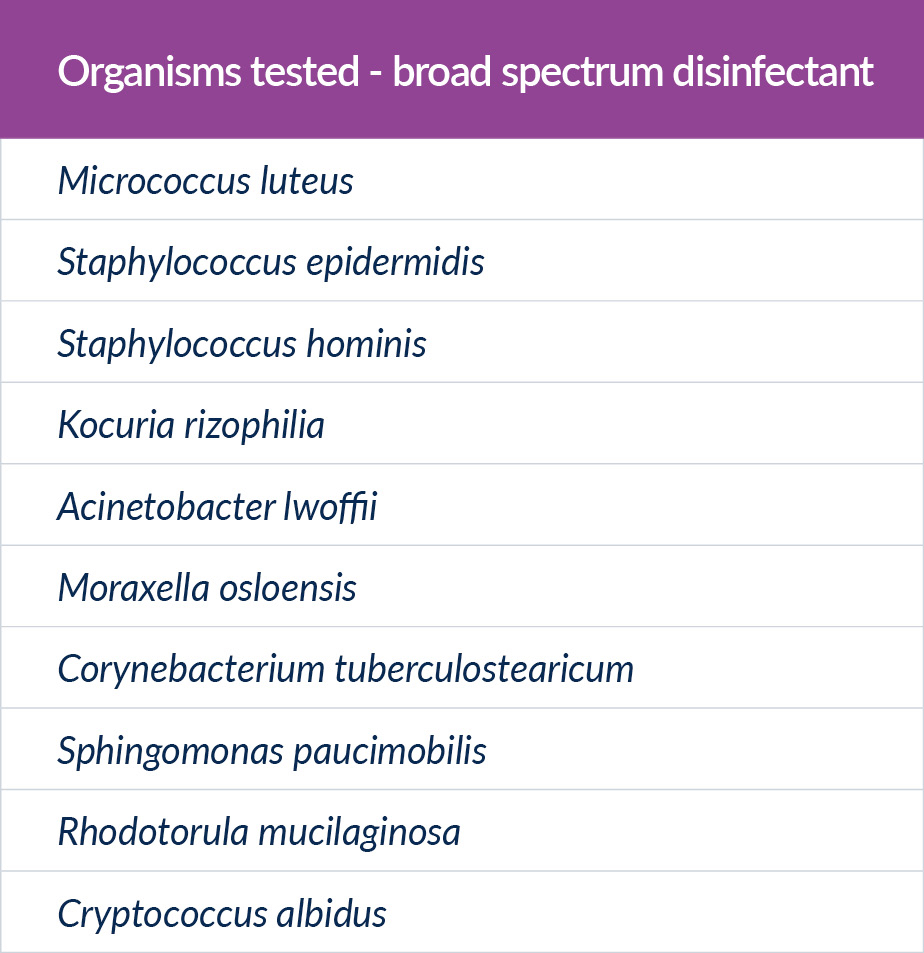
Figure 3: Organisms tested
We set our own acceptance criteria, which differed slightly from the non-industry specific EN tests. This was mainly based on the much lower expected contamination levels found in life science cleanroom and we chose to follow USP <1072> Disinfectants and Antiseptics guidance of a log 3 reduction in bacteria and a log 2 reduction in fungal and bacterial spores. We tested a sporicide and a broad-spectrum disinfectant, based on hypochlorous acid, with a contact time of 1 min as a sporicide and a 3 min contact time for the broad spectrum disinfectant.
Surface test results
The results of the test were fascinating as they clearly showed that the expectation of the regulator that disinfectants should be tested on all key surfaces, not just stainless steel is valid as the results changed significantly depending on the combination of organism and surface. Interestingly both disinfectants would have passed against all organisms on stainless steel but failures against our acceptance criteria were seen on PVC flooring samples, isolator canopy materials, glass and aluminium.
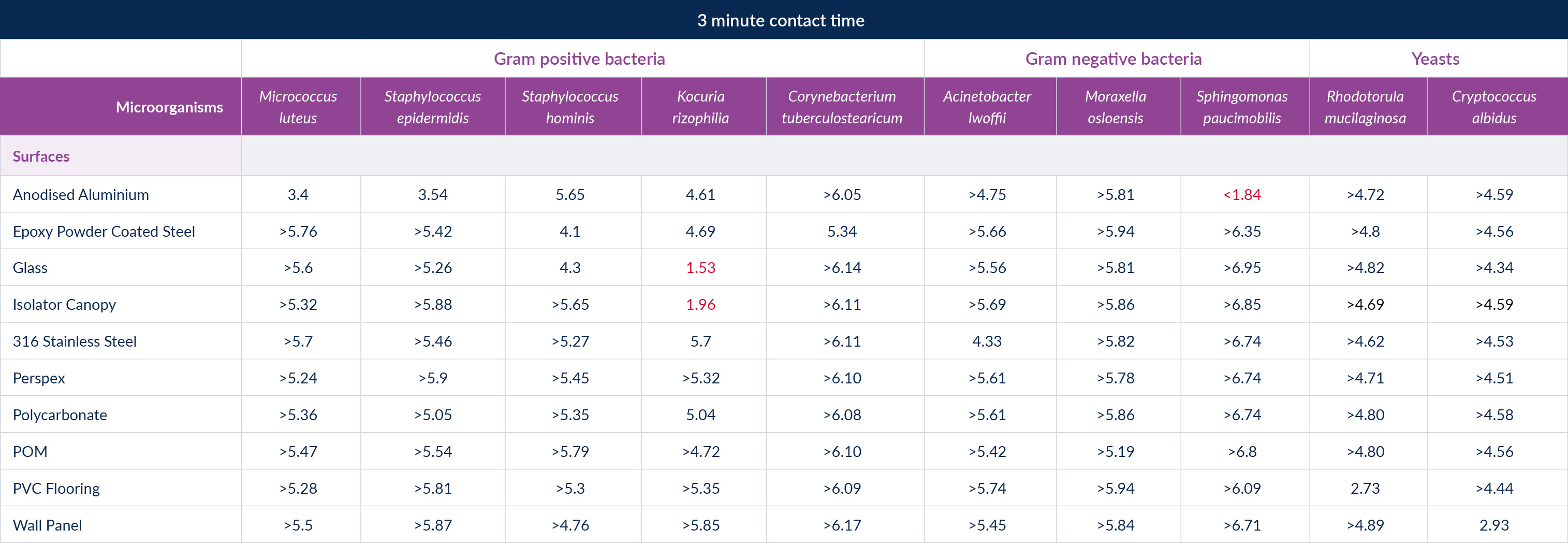
Figure 4: Broad spectrum disinfectant test results
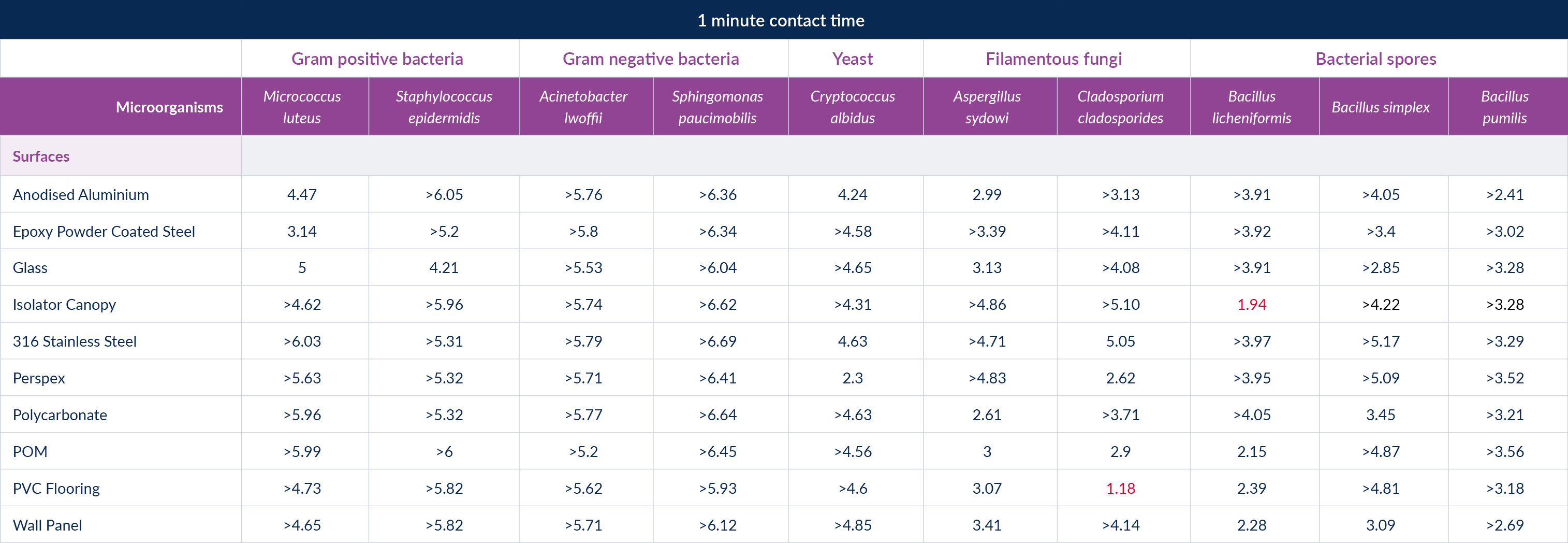
Figure 5: Sporicidal disinfectant test results
If you read along the rows, you can see the difference the organism makes on the same surface, not always following the accepted hierarchy of easiest to hardest to kill is bacteria, yeasts, fungi and bacterial spores.
If you read down the columns you can see the difference the surface makes on how easy the organism is to kill, with passes not being achieved on all surfaces for the same organism.
Check the condition of the samples given to the lab, are they truly representative of the surfaces in the cleanroom or is it damaged or scratched?
However, it must be remembered that failure to achieve acceptance criteria in a one-off test may not mean your disinfectant has “failed validation. Consideration should be given to the number of replicates carried out as test results can vary significantly between tests. Check the condition of the samples given to the lab, are they truly representative of the surfaces in the cleanroom or is it damaged or scratched? If consistent failures are seen, can the surface be replaced in the room, not always feasible but it could possibly be a solution. Were the acceptance criteria set really representative of the process of cleaning and disinfection in the rooms, consider modifying contact time, starting inoculum levels, interfering substances used, to ensure the EN test is more closely representative of a cleanroom situation. If the disinfectant is always applied with mechanical action, compare with an EN16615 test result. And most importantly, reach out to your manufacturer.

