A shutdown is a well-known concept within many production facilities of various kinds. In principle, every company is affected by a temporary shutdowns and there is virtually no possibility of carrying out a planned shutdown without an economic consequence. After all, employees are not able to work normally, so there is no output and the activities that are carried out usually cost a lot of money.
Let us not confuse the planned shutdown with an incident leading to a lockdown of part or all of production, which is an entirely different situation.
Recurring shutdowns are seen in many industries in order to carry out preventive maintenance and, where appropriate, smaller corrective projects. However, if we focus on our area of expertise “Controlled Environment” (CE), the objective and content of a shutdown is clearly more complex compared to most general industrial sectors. In contrast to general industry, the shutdown within the CE market is also often an annual operation. In a CE facility it is primarily about the concept of contamination. This contamination can consist of dust or fibres, airborne microorganisms, fine dust or chemical vapours.
The entire implementation process and record should be in a comprehensive ‘Shutdown Management Plan’
We try to create an environment in which contamination to the product process or research can be limited, or if possible prevented completely. In addition, there is often a controlled climate (temperature and RH) in such areas in order to be able to carry out the specifically planned activities.
For the sake of convenience, let us use the umbrella term 'cleanroom' when we talk about these spaces. Of course, an OR in the hospital, or high-care area in the food industry, is also a facility in which the exclusion of contamination plays an important role, yet you do not say that you will be transported to the 'cleanroom' if your tonsils need to be removed.
In this article, we will try, among other things, to clarify why a shutdown in a production facility with cleanrooms differs from other industries.
Organising a planned shutdown
In the case of a shutdown of a somewhat larger production company, of a general nature, a specialised facility manager or maintenance manager will usually start by putting together an experienced shutdown project team. This project team should first of all freeze the schedule of the shutdown period.
Starting time and expected duration are important factors for all other stakeholders within the organisation.
Needless to say, clients are not allowed to notice anything or very little of this necessary evil. Sales and logistics, among other things, must also remain well informed about the shutdown plans and its progress. Therefore, if external communication to customers is necessary, the sales management will have good and up-to-date information!
The shutdown team will then prepare, plan and coordinate the entire implementation process and record all related information in a comprehensive Shutdown Management Plan (SMP). Obviously, the team will also be responsible for monitoring and evaluating the entire process during and after the shutdown.
Shutdown within the controlled environment market is often an annual operation
They start by determining whether all planned work actually requires a shutdown. If this is not the case, an activity that is not necessary is scheduled for during normal operational maintenance times. The team will then usually consult the evaluation of the previous shutdown to learn from. This is followed by the collection and study of up-to-date information about the site, normally documented in an updated Site Master File with all attachments that may be relevant in relation to legislation and regulations as well as the possible risk assessment carried out. The Master Validation Plan and the Calibration Plan may of course not be omitted during the preparation.
Ideally, all the files mentioned are included in a Document Management System (DMS software) and the maintenance planning is implemented in the CMMS (Computerised Maintenance Management System) on the basis of an LTMP (Long-term Maintenance Plan). If such software systems are not available, it will have to be manually traced and clearly registered in order to be able to follow the planning flexibly and adjust it where necessary during the execution of all activities.
Possible internal optimisation programmes such as an Overall Equipment Effectiveness (OEE) programme as an important part of a Total Productive Maintenance (TPM) care system can also play a role in the preparation of the SMP.
When talking about a general factory, the shutdown maintenance programme usually concentrates on the following activities:
- The planned maintenance of the equipment
- The systematic maintenance of the structural interior (floors, doors, etc.)
- Planned maintenance of general HVAC equipment (filters, etc.)
- Systematic periodic work on the infrastructure of the building
- Planned calibration of equipment, measuring and monitoring equipment, etc.
Of course, the work mentioned above, which is carried out during a shutdown, has been evaluated and it has been established that it is impossible to carry out this maintenance during normal operational functioning of the plant. That should be clear. Never forget that every day without production costs an enormous amount of money!
In addition to maintenance, minor modifications or renovation work will be carried out, which can only be carried out when production has been completely (or partially) halted.
It is useful to establish at the start of the plan who will be responsible for the internal communication of notifications to staff and communication to other external stakeholders.
The shutdown in a controlled environment
The CE market can primarily be divided into the hi-tech (related) segment and the pharmaceuticals and life sciences one. In addition to this rough two-part division, the food sector should be mentioned as the third segment. Within this sector there are many developments around the theme of food safety and many changes in legislation and guidelines at a global level. More and more one can speak of a CE facility within this market segment.
The controlled environment market can primarily be divided into the hi-tech (related) segment and the pharmaceuticals and life sciences one
Independently of the market segment in which one is active, the general shutdown activities mentioned above will also apply as a basis for production facilities in which 4 cleanroom spaces are built. The definition of activities will often be regulated on the basis of an existing ISO 9001 quality management system.
In addition to this basic task, there is clearly more being demanded from the shutdown team as a result of the more extensive legislation and regulations within the CE sector.
The hi-tech market
These more extensive requirements in relation to maintenance, cleaning and annual validation are often based on the specific ISO 14644 standard as well as sector-specific (VCCN) guidelines. Of course, there are also requirements for the applied cleanroom related to the URS (User Requirements Specification). (VCCN: Dutch national member organisation of the ICCCS (International Confederation of Contamination Control Societies)).
The requirements for the design, construction and start-up of a dust- and germ-free room are described in the ISO 14644-4 standard, but this part of the standard also clearly describes what is required for maintenance activities, performance monitoring as well as documenting the whole of the equipment, the construction facility and the complete technical installation, in the broadest sense of the word.
The 14644-3 standard describes the tests that are important for the initial qualification of the cleanroom. However, the standard also requires that, during the use phase, proof is provided that the room continues to meet the initial process qualification as established upon commissioning.
Attention: The standards mentioned often describe what needs to be done, but not how! It is up to the entire management team of an organisation and the EHSQ team (Environment, Health, Safety and Quality) to determine how all internal (policy) matters and tasks as well as Standard Operating Procedures (SOP) are carried out in order to demonstrate that everything that is required is indeed met.
In the high-tech (related) market there is normally a check on compliance with agreements made and compliance with agreed standard standards by means of internal or customer Audits. The government primarily pays attention to municipal or provincial legislation and regulations. (Environmental permits or licences, etc.). For ISO 14644 standardised cleanrooms within the high-tech (related) cleanrooms, the repetition cycles from Table 1 apply during the usage period.
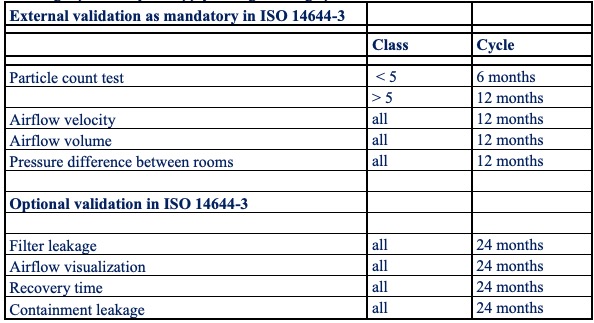
Table 1
GMP related market
Within the pharma, life sciences and food markets, companies in the US must comply with Good Manufacturing Practices (GMP). GMP is a quality assurance system aimed at creating a safe production process around products related to human and animal health.
The GMP standards have been framed within the legislation by the Food and Drug Administration, or FDA. The FDA is the agency of the US federal government, which controls the quality of food and medicines in the broadest sense of the word. The FDA also controls the treatment of blood, medical products and cosmetics.
In a, where desired, slightly modified form, these GMP standards are laid down for Europe in the EudraLex, which stands for the collection of rules and regulations for e.g. medicines in the EU. Incidentally, EU legislation is applied in Europe for the various market segments mentioned above, in which the GMP regulations are often also used as a basis. For exporting food products to the USA, an implemented GMP care system is necessary (CFR Title 21 part 117).
It is clear that the regulations, as mentioned above, are clearly stricter than those that apply to the high-tech market. The licence, which is necessary in order to carry out production activities in the GMP related market segments, can only be issued if the requirements for this quality assurance system are met.
Table 2 illustrates an overview of the validation measurements and repetition cycles during the usage period.
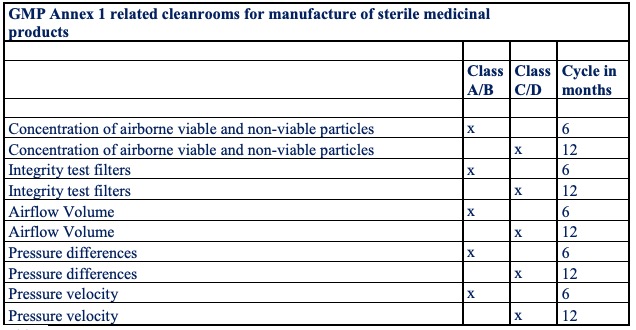
Table 2
In the Netherlands, the Health Care and Youth Inspectorate (IGJ) issues a GMP certificate to the manufacturer if it complies with the GMP guidelines. The IGJ carries out periodic inspections of manufacturers in the Netherlands to verify whether they comply with the GMP regulations.
If the GMP rules are not met, the GMP certification is withdrawn and the company loses its production licence.
The IGJ also inspects manufacturers in and outside Europe on behalf of the European Medicines Agency (EMA) and the Medicines Evaluation Board (MEB). In addition to the above validation measurements, the GMP standard also refers to a continuous monitoring process to determine that the effective operation of all technical and structural components is adequate. An LTMP is required and the monitoring of the performance should be documented, as well as the documentation of the entire equipment, the structural facility and the complete technical installation, in the broadest sense of the word.
Not only during a shutdown period, but also after the implementation of any change or maintenance activity of a significant nature in the composition of the equipment, the HVAC installation or the structural cleanroom, a full validation of the above requirements, as listed in table 2, should be carried out. Risk Assessments are performed during cleanroom development and maintenance, to identify, assess, reduce/eliminate (where applicable) and control contamination risks. It is clear that the planning and timing of a shutdown in cleanrooms with GMP quality assurance clearly differs from that in general production facilities or cleanrooms within the high-tech sector.

