In vitro testing is performed to evaluate the efficacy of disinfectants in the laboratory under simulated cleanroom conditions. In vitro tests potentially consist of suspension studies and coupon studies. Suspension studies are primarily used for screening purposes and do not consider the various cleanroom surfaces.
A suspension test also presents a significantly lesser challenge to disinfection than a surface coupon test, with 360 degrees of potential disinfectant access to the cell, and does not evaluate any potentially challenging interactions between the microorganism, the disinfectant, and the surface material.
It is important to understand the hierarchy of microorganism challenge to chemical inactivation and the efficacy profile of the disinfectants to select appropriate microorganisms and an appropriate wet contact time
A coupon study is more representative of the actual cleanroom conditions, as the test involves using various cleanroom substrates with site isolates (if possible) to simulate the actual cleanroom conditions including the disinfectant wet contact time. Once the in vitro study is completed, the end-user can start to use the disinfectants in the GMP cleanrooms and conduct in situ testing.
The in situ testing phase is where the efficacy of the disinfectants are evaluated in the actual cleanroom environment. This testing demonstrates the effectiveness of the products, wet contact time, and application procedures in the “real world.”
A coupon study is more representative of the actual cleanroom conditions
The following regulations and guidance documents are related to disinfectant validation:
FDA Guidance for Industry - Sterile Drug Products Produced by Aseptic Processing, Current Good Manufacturing Practice (2004)
“The suitability, efficacy, and limitations of disinfecting agents and procedures should be assessed. The effectiveness of these disinfectants and procedures should be measured by their ability to ensure that potential contaminants are adequately removed from surfaces.”
USP 43 <1072> Disinfectants and Antiseptics
“The selection of suitable disinfectants and the verification of their effectiveness in surface challenge testing is critical in the development of a cleaning and sanitization program.”
Annex 1 – Manufacture of Sterile Medicinal Products (Aug 2022)
“The disinfection process should be validated. Validation studies should demonstrate the suitability and effectiveness of disinfectants in the specific manner in which they are used and on the type of surface material, or representative material if justified, and should support the in-use expiry periods of prepared solutions.”
The following are warning letters (GMP Trends, 15 Sep 2021. CI Issue# 1072) related to disinfectant validation:
- “The study was performed using only ….. No other typical USP growth promotion microorganisms, and especially no in-house isolates, were evaluated in this study.
- The study included ….. No other surfaces present in the cleanroom were evaluated.
- There was no analysis to determine if the microbial population used consisted of spores and/or dividing vegetative microorganisms.”
Points to Consider for Coupon Studies
Coupon studies are the most critical aspect of an in vitro study, as coupon testing simulates the actual cleanroom conditions, from a scientific and regulatory perspective. This simulation involves using actual cleanroom surfaces and in-house isolates in the coupon tests.
The following are points to consider for coupon study:
- Efficacy of disinfectants
- Wet contact time
- Selecting the appropriate microorganism type
- Selecting the representative surfaces
Efficacy of disinfectants: The first step is to understand and carefully consider the various types of chemical agents and design an appropriate cleaning and disinfection programme for the cleanroom. As per United States EPA (Environmental Protection Agency) classification, antimicrobial agents can be classified into three broad categories: sanitisers, disinfectants, and sporicidal agents.
A sanitiser is expected to reduce the level of bioburden. A disinfectant is expected to inactivate vegetative microorganisms, some mould spores, and viruses. USP<1072> defines a chemical disinfectant as “A chemical agent used on inanimate surfaces and objects to destroy infectious fungi, viruses, and bacteria, but not necessarily their spores”.
In contrast, a sporicidal agent, which is typically an oxidising agent, when used with sufficient concentration and wet contact time, is expected to inactivate the range of microorganisms encountered in a cleanroom, including vegetative microorganisms, moulds, viruses, and fungal and bacterial spores. USP<1072> defines a sporicidal agent as “An agent that destroys bacterial and fungal spores when used in sufficient concentration for a specified contact time. It is expected to kill all vegetative microorganisms.”
The contact time can vary depending on the type of microorganisms, the substrates, and the disinfectant’s efficacy. This measurement is also known as the wet contact time, often 10 minutes for disinfectants and sporicides (alcohol-based products are generally 1 minute). Coupon studies verify the effectiveness of the wet contact time.
Selecting the appropriate microorganisms: A broad spectrum of microorganisms that include Gram-positive, Gram-negative, vegetative form fungi, and fungal and bacterial spores are typically selected for the coupon studies. The use of facility isolates is also expected from a regulatory perspective. Examples of typical challenge microorganisms are shown below:
- Gram Positive Cocci: Staphylococcus aureus
- Gram Negative Bacilli: Pseudomonas aeruginosa
- Mould: Aspergillus brasiliensis
- Yeast: Candida albicans
- Bacterial spores: Bacillus cereus, Bacillus subtilis (bacterial spores are only for sporicidal agent challenge)
Understanding the microorganism’s chemical disinfection challenge characteristics and the efficacy profile of the disinfectant is essential for selecting microorganisms. Bacterial spores are more difficult to kill than fungal spores and vegetative bacteria. This knowledge will help choose the appropriate microorganisms for the challenge to a specific disinfectant, and guide appropriate use within a facility.
For example, if a bacterial spore is used in a coupon study with a disinfectant such as a Quaternary Ammonium Compound, a failing result will be observed; as Quaternary Ammonium Compounds are not expected to be effective against bacterial spores. They are typically the most challenging form of microorganism to be tested in an in vitro study and also to inactivate in a facility.
Bacterial spores should be tested in a coupon study against a sporicidal agent. Hence, an understanding of disinfectant efficacy and selecting appropriate microorganisms will facilitate successful coupon study outcomes.
Selection of representative substrates
Disinfectants can have varying efficacy on different cleanroom surfaces. It is required to include the various types of cleanroom surfaces in the coupon study. However, there may be many different kinds of surfaces in a cleanroom, and it may not be feasible to include every type of surface in a coupon study. A common question is, how to best choose representative cleanroom surfaces for a coupon study?
How to best select representative cleanroom substrates is a commonly asked question for a disinfectant efficacy study. Best practice is to perform a risk assessment to select the representative cleanroom substrates, make scientifically sound selections, and reduce the amount of testing required.
An example of risk classification and assessment is shown in Tables 1 and 2, respectively. From Table 1, the risk parameter in consideration includes the following:
Surface roughness: Typically, the higher surface roughness will pose a greater challenge in the cleaning and disinfection process.
Frequency of contact: Surfaces with a high frequency of contact will likely have a higher bioburden load.
Percentage surface area of the system: This is the proportion of the substrate in the specified area/system.
Proximity to critical process: The area is near a critical process, such as when products or sterile parts/packaging components are exposed to the environment.
These risk parameters can be classified into three different risk categories (Low, Medium, and High) with corresponding risk scores based on the description shown in Table 1.
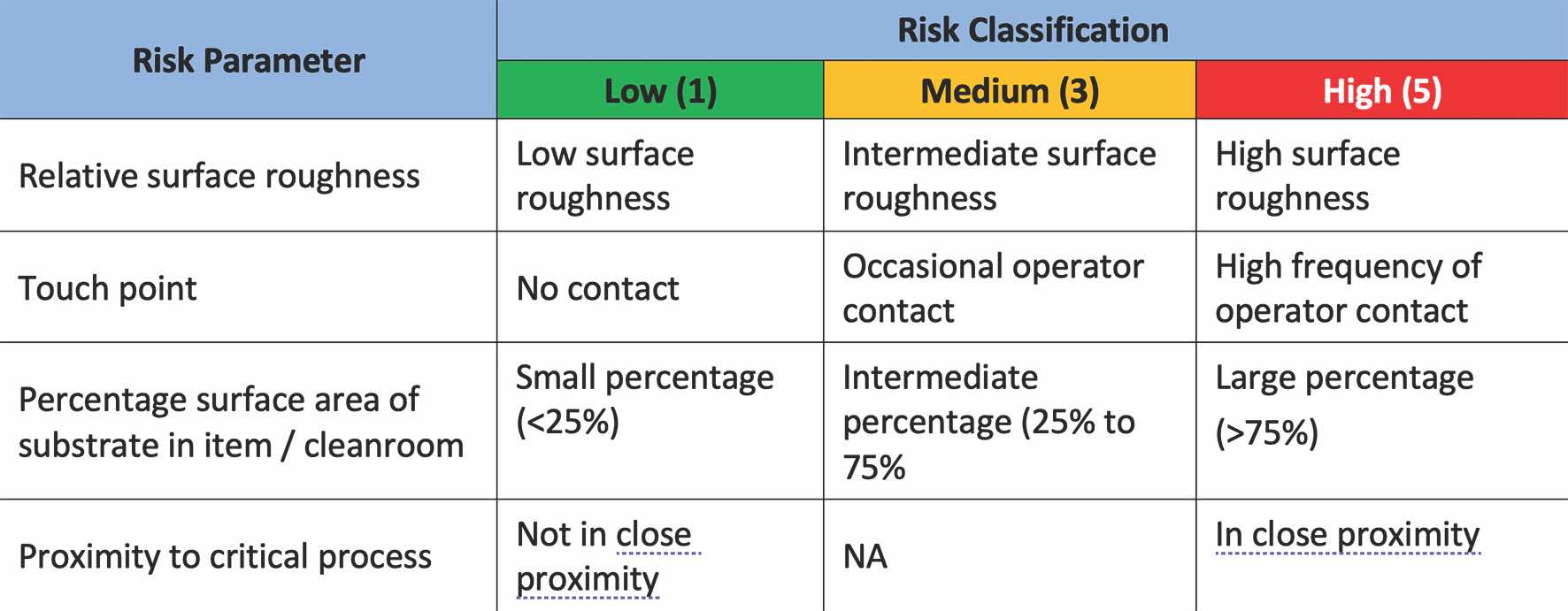
Table 1: Risk parameters and classification of substrates
Risk assessment is performed on different substrates from different areas and systems according to the risk parameter defined in Table 1. The Risk Priority Number (RPN) is calculated by the product of the numbers assigned to the four risk parameters, as shown in Table 2.
Based on the risk assessment in Table 2, determining the representative substrates for cleanrooms, furniture, and laminar flow hoods is based on the relative significance of the Risk Priority Number (RPN) in each category. From the risk assessment, the terrazzo tiles, powder-coated galvanised steel sheets, and glass can be chosen as the representative surfaces for the cleanroom based on the relatively high RPN. For the furniture, stainless steel 304 L is the representative surface, and for the laminar flow hood, both stainless steel 304 L and glass can be chosen as the representative surfaces.
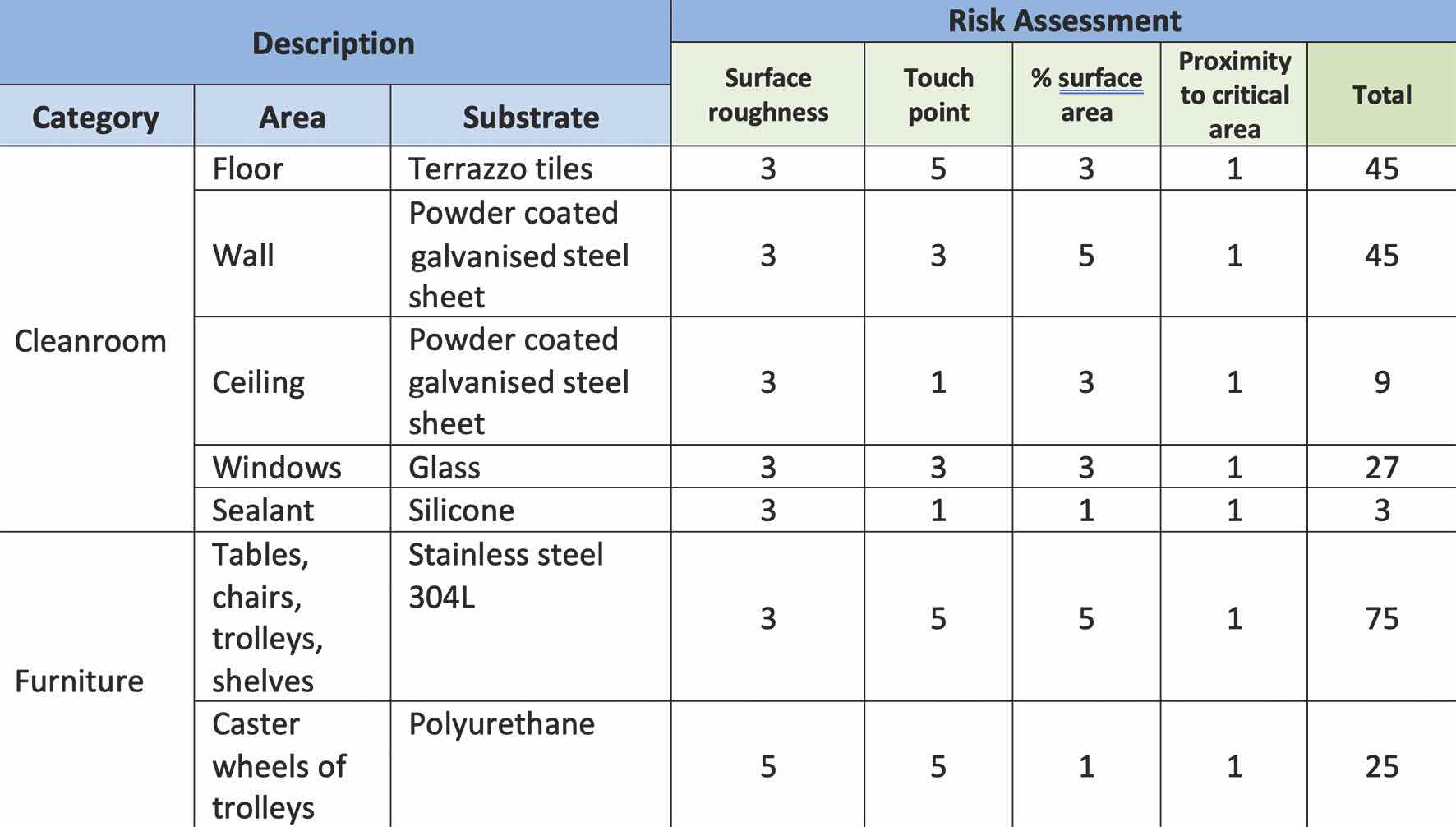
Table 2: Risk assessment

Table 2: Risk assessment
Conclusion
An in vitro coupon study is a laboratory study to evaluate the efficacy of disinfectants under simulated cleanroom conditions, against site isolates and potentially, some degree of reference strain microorganisms on representative cleanroom substrates.
It is important to understand the hierarchy of microorganism challenge to chemical inactivation and the efficacy profile of the disinfectants to select appropriate microorganisms and an appropriate wet contact time. In addition, representative cleanroom surfaces are best selected by utilising a risk-based approach.
All these considerations are critical to the success of the coupon study.





