The optimal airflow is an important factor in contamination control in a cleanroom and is the single largest factor in determining the size of the air handling system. Many air change rate (ACR; or air changes per hour - ACPH) recommendations were intuitively developed decades ago with little scientific research to back them up and operate very far above the levels required for regulatory compliance.
This also equates to up to 75% of a facility's energy costs and has today moved into the focus of energy and CO2 reduction efforts.
Air change rates per hour - a regulatory requirement?
The short answer is: NO. In commercial, home air conditioning, and ventilation systems, the volume flow depends on the needed thermal power of cooling and/or heating, allowed temperature range, amount of outdoor and recirculating air, filtering out particles and/or odours, air movement in the occupied area, and some other specific parameters like flushing out pollutant.
When only mechanical ventilation is possible and people are constantly present, local laws specify a minimum outdoor air rate per person – this is often mixed up with the wording “air-change-rate” used in cleanrooms.
All this may sound very complicated but it is the technical standard of good engineering practice (GEP) and is determined by laws of physics.
Are set air change rates per hour a regulatory requirement? The short answer is: NO
High-tech components and equipment are manufactured and assembled “dust-free” in cleanrooms. Drugs for human and veterinary use are also manufactured in cleanrooms. They shall not pose any risk to patients and should be (as less as possible) free of pollutants of any kind. Particularly higher risk is associated with parenterally delivered substances. Knowing this, you would think that clean air ventilation in cleanrooms should be somewhat more complex in determining the needed air volume flow. But, up to date, it is handled much easier!
For more than decades, there has been the conviction that a fixed ACPH for each cleanroom class and a flow velocity of 0.45 m∙s-1 for an EU-Grade A (ISO5) is “a regulatory requirement” and therefore no detailed calculation has to be done.
Unfortunately, the “nonbinding recommendation” of the FDA Guideline for Industry [1] that is seen as a requirement.
This misinterpretation is common practice and the result is far excessive air flow rates causing an energy demand for cleanrooms that accounts for up to 75% of the energy costs for a production site.
Determination of air volume flow in cleanrooms
In addition to the calculation of air volume flow for home and commercial needs, cleanrooms require some more detailed considerations. These are but are not limited to, flushing out and dilution of generated particle matters as non-viable (NVP) and microorganisms (VP, viable – bacteria, yeast, mould) from personnel and processes.
Outdoor air rate is required for personnel demands, for the dilution of max. permitted concentration of chemical and biological substances, and supplementation of exhaust air and overflow caused by room pressure cascades.
Therefore more parameters than only for thermal need have to be calculated to determine the air volume flow according to the air cleanliness class needed. GEP gives the equation E1 for thermal load calculation needs:
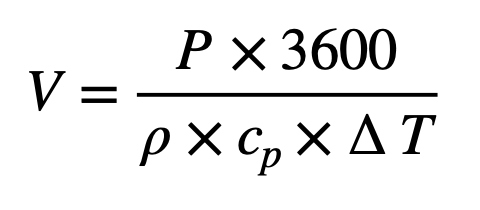
Where V [m³∙h-1] is the calculated air volume flow, P [kW] is the thermal load in heating or cooling, ρ [kg∙m³] is the air density (approx. 1.2 at 20°C at 1013 mbar, relative humidity of 30% – 60 %), and cp [kJ∙kg-1∙K-1] is the specific heat capacity of air (K for Kelvin-degree = °C + ~273). ΔT is the allowed difference in air temperature between supply air and room temperature (typically 4 to max. 8 K in cleanrooms).
This misinterpretation is common practice and the result is far excessive air flow rates
The required air volume flow rate (V in m³∙s-1 according to ISO 14644-4:2022 [2]) to maintain a specified concentration limit for particles is determined by the rate of particles emitted in the cleanroom (source strength S in total particles emitted per second - P∙s-1), the ventilation effectiveness ε, and the allowed limit of total particles CL (P∙m-3) and will be calculated by equation E2.
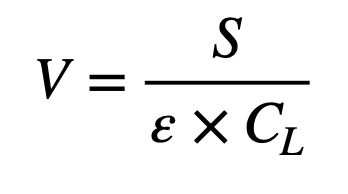
This equation assumes that the number of particles entering the cleanroom or clean area from the supply air is negligible and can be omitted from the equation. This is a quite possible assumption together with adequate multi-stage filtration (e.g. ePM1 >50% + ePM1>85% according to ISO 16890 [3]) and a final HEPA-filter specified to an international standard (e.g. ISO35E according to ISO 29463 [4] – efficiency ≥ 99,95% at most penetrating particle size MPPS). The ventilation effectiveness ε is also described as the Contaminant Removal Effectiveness Index (CRE) and can be calculated with the equation E3.
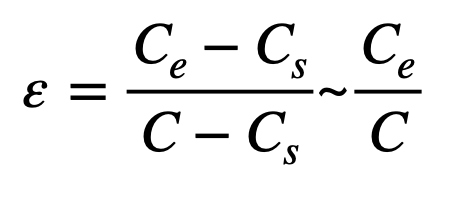
Where ε is the CRE-index, Ce is the particle concentration in the exhaust or return air, Cs is the particle concentration in the supply air – negligible low in cleanrooms - and C is the average particle concentration at critical points in the cleanroom or clean area. The CRE index is strongly dependent on the airflow and thus on the position and type of the supply and exhaust grids and also the furnishing of the cleanroom.
Supply and exhaust grids in the ceiling have a certain bypass effect which reduces the ventilation effectiveness (Figure 1). Exhaust grids installed close to the floor increase efficiency (Figure 2), whereas exhaust via a false floor would be an even better solution.
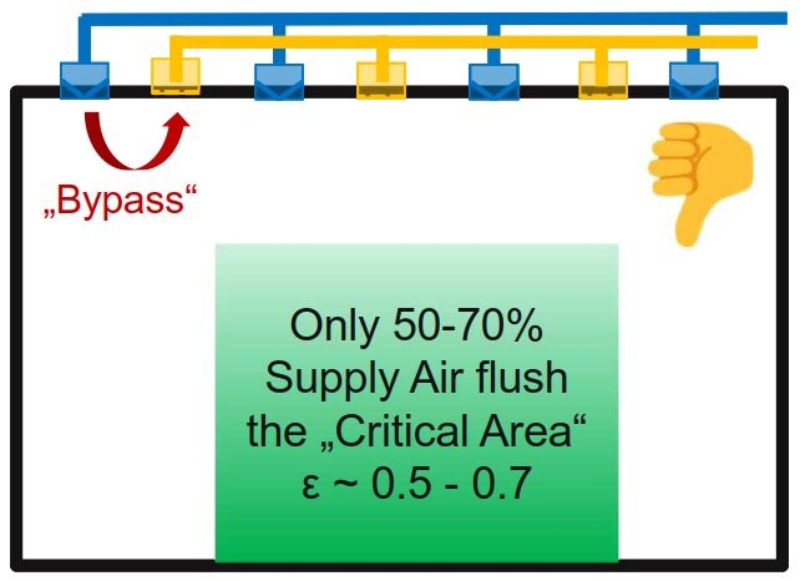
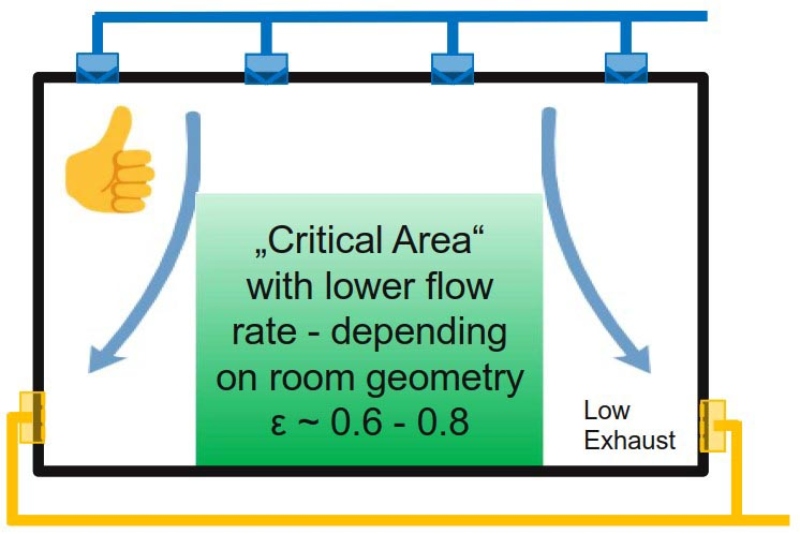
A false floor is not desirable in the pharmaceutical industry for various reasons (e.g. wet cleaning). The allowed number of particles CL in E2 can be set according to the class limit of ISO 14644-1 [6]. For safety reasons, the factor CL can be determined at a lower limit, e.g. if “max. 30% of the limit of ISO 14644-1” should be allowed. The ISO series of standards is valid for all types of cleanrooms. Part 1 deals with air cleanliness according to total particle concentration. The total particle concentration counted by a Light Scattering Airborne Particle Counter (LSAPC) includes all airborne suspended particles like NVP and VP.
In pharmaceutical cleanrooms, you have to differentiate these types of particles. To calculate the air volume flow depending on VP you can also use the equation E2 by using the corresponding values of source strength and limit with or with no “safety factor”.
Sounds easy but is not as easy as it looks. The biggest challenge in calculating airborne particle matters is the uncertainty of source strength. Sources of airborne contaminants are very different and could be, but are not limited to;
- Personnel with gowning and behaviour
- Personnel and material flow
- Material and packaging components
In various literature, you can find information about the total particle and germ emissions of personnel. The company Dastex has already made measurements of personnel in different clothes and activities in a so-called “Body Box”.
So far so good, does it correspond with practice?
From all the above-mentioned equations, you will get out three values of the air volume flow by source:
- thermal load (E1)
- total particle load (E2)
- VP load (E2)
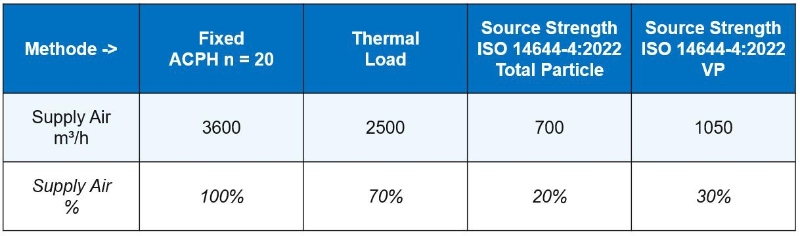
The largest value from these three calculations is the required air volume flow rate for your cleanroom.
Table 1 shows an example calculated in a pharmaceutical cleanroom Grade C in operation (ISO8) with a length of 10m, a width of 6m, a height of 3m (volume of 180 m³), and a high thermal load of 5 kW and 4 persons in cleanroom clothing (coverall, hood, face-mask, gloves, booties).
The total particle limit was set at 30% of the allowed class limit (1,056,000). The total particle source strength of particles ≥ 0.5 µm for each person was assumed at 35,500 per second – compared to body-box results of 1,800 per second this is about 20 times higher – and 5 VP per second from the maximum value of body-box testing.
Some companies have conducted trials with air volume flow reduction in cleanrooms
Within the last few years, some companies have conducted trials with air volume flow reduction in cleanrooms and all of these results show, that the determining value in most cases is the thermal load.
Behrens et al. have done an experimental study to find out to what extent the fixed values of a 20- or 10-fold ACPH can be used to comply with the limits for total particles and colony-forming units (CFU) according to the FDA guideline and the EU GMP Annex 1:2022 for a grade C Cleanroom. The result shows that even an ACPH of 10 would support a grade B area within the limits of total particle load and VP∙m-3.
The EECO2 Group (www.eeco2.com) has shown that an ACPH rate of 2-fold in a GMP-grade C room is sufficient to hold the particle concentration at a maximum of 30% of the allowed limit of the current EU-GMP-Annex 1:2022 whereas the thermal load requires a 5-fold ACPH. The FDA is today the only organisation that still recommends the value of a 20-fold ACPH in its 2004 guideline.
With the knowledge of today, this recommendation is by no means any more state-of-the-art. The effectiveness of lower ACPH is already confirmed by implementation in operational cleanrooms.
