INCOG BioPharma Services (INCOG), an Indiana-based contract development and manufacturing organisation (CDMO), has reached another major milestone in its facility by completing the construction of its cleanroom production area within the new 90,000 sqft biopharma manufacturing site.
For the major project in the city of Fisher, INCOG chose AES Clean Technology (AES) for a modular solution. Speaking about other partners, INCOG's VP of Operations, Alex Haig, who steered the project, added: "I'm thankful for our partners at AES, Shiel Sexton, Javan Engineering, OPTIMA Pharma, and extended teams."
The fill-finish organisation will be a key partner to life sciences companies that outsource the manufacturing of innovative injectable therapies.
INCOG has utilised an integrated design-delivery approach throughout the project to design, install, and commission its cleanroom in record time
The newly completed cleanroom area within the building will feature a multi-use aseptic filling line, including fully integrated isolator technology, that can process both bulk vials and ready-to-use (RTU) vials, syringes, and cartridges. In addition, INCOG plans to conduct formulation activities, inspection, labelling, and packaging services in its recently constructed manufacturing space.
INCOG partnered with AES, a provider of cleanroom facilities supporting the biopharmaceutical industry. AES equipped the new state-of-the-art facility in Fishers with its modular cleanroom systems and high-performance HVAC technology to optimise environmental control. The recently installed cleanroom systems provide INCOG with the flexibility and customisation for growth, including the ability to quickly triple its drug product manufacturing and handling capacity via future capital investment projects.
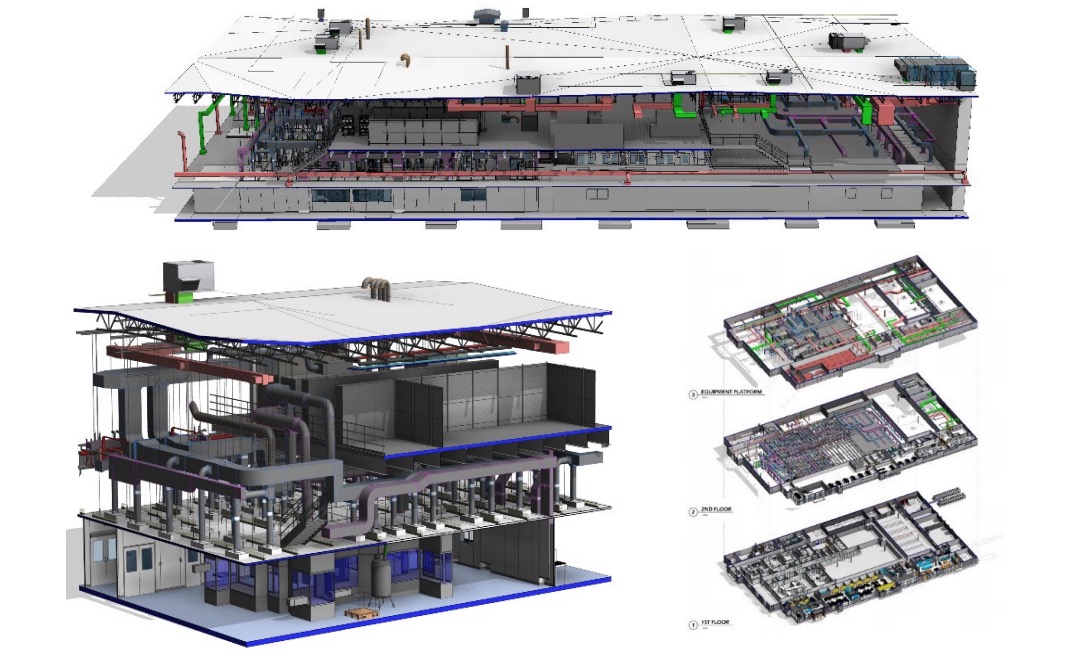
Cross sectionals of AES’s modular cleanroom technology (installed at INCOG BioPharma’s Fishers, Indiana facility)
With both speed-to-market and impact on patients in mind, INCOG has utilised an integrated design-delivery approach throughout the project to design, install, and commission its cleanroom in record time. AES has helped drive the process toward successful commissioning and qualification at every step of the way.
Grant Merrill, CEO of AES Clean Technology said: "We are proud to partner with INCOG and support their mission of creating a better path to market for life-saving drugs through the therapies that they manufacture inside our cleanroom facilities."
"INCOG is now even closer to opening our doors to advance therapies into the hands of patients and providers. The completion of the cleanroom space means we are ready to install our OPTIMA filling line and maintain our target to be CGMP ready by Q3 2022. With growing demand for injectables, our team is well positioned and ready to help alleviate capacity constraints in the global markets."
The completion of the cleanroom space means we are ready to install our OPTIMA filling line
INCOG has assembled a team with deep domain experience and knowledge in the formulation, development, and manufacturing of injectable medicines. The company has hired nearly 50 people in the last several months and plans to add another 50 positions by the end of 2023.
Recently recognized as a "Best Place to Work" by the Indiana Chamber of Commerce, INCOG provides an entrepreneurial culture that fosters collaboration and promotes genuine teamwork.

