Sola dosis facit venenum: "Only the dose makes the poison". The quote from Paracelsus, the Swiss physician, alchemist, and astrologer born in 1493 —considered by many as the father of toxicology— posits that any substance can become a poison at certain doses. The problem arises when a drug, which is formulated to cure, becomes precisely the opposite: a poison.
Shockingly, data from the FDA show that the main causes of injectable drugs product recall are the presence of particles, lack of sterility and bacteria contamination
Both professionals and companies involved in drug manufacturing are therefore part of the solution and have the responsibility of improving the situation, either by designing safer facilities, investing in safer equipment or manufacturing in a safer way.
This article compares two technologies used for mitigating contamination risks in the manufacture of sterile medicines through aseptic process (isolators and RABS), identifying main differences and uses depending on the product and type of application, and give a glimpse of the trends in the field of track-and-trace system integration into aseptic and sterile processes.
EU GMP Annex 1
The acronym RABS appears 19 times in the new draft of Annex 1, but there is no mention in the current version. The draft also recommends the use of both terms, RABS and isolators, clearly confirming them as a trend.
RABS, short for restricted-access barrier systems, isn’t just a combination of physical barriers (gloves, glass surfaces, etc.) with a unidirectional airflow pattern or laminar flow, but a setup focused on reducing the risk of product contamination. These measures include monitoring systems for both viable and non-viable particles, validated manual or automatic decontamination systems, the opening of barriers only in exceptional cases and the recording of each event through a risk assessment, plus other SOPs for the entry and exit of materials.
RABS have distinct design characteristics, which according to the ISPE are:
- Rigid wall enclosure
- ISO 5 unidirectional airflow in the critical area (ISO 7 in the room in operation)
- Sterilisation-in-place (SIP) for parts in contact with a liquid product, if possible
- Sterilisation in autoclave and aseptic assembly (piece of tubing product)
- Autoclave sterilisation for parts in contact with the product
- Materials entry through transfer systems that prevent the exposure of sterile surfaces to less clean classification environments
- Use of glove ports
- Disinfection of all parts without direct contact before each batch
RABS can be classified as passive systems when using the existing HVAC in the room, or active when equipped with built-in fans. They can also be classified as open RABS with airflow overspill to an ISO 7 or ISO 5 background, or closed RABS including closed ducted systems. They are also classified according to a leak rate.
An important point to consider in the design of an aseptic product line is how the transfer of materials takes place: the objective is to minimise the time of transfer and/or exposure to areas of lower classification thus mitigate the risk of contamination.
The objective is to minimise the time of transfer and/or exposure to areas of lower classification thus mitigate the risk of contamination
There are three types of transfer devices:
- Mobile laminar flow carts or closed containers
- Bags or containers previously sterilised
- Decontamination in-situ just before entering the critical area into the RABS (e.g. pre-chamber with decontamination with H2O2)
Isolators for aseptic zones
Unlike a RABS, an isolator doesn’t necessarily have to be located within an aseptic zone. These units are so-called containment isolators, and their mission is to contain the product, preventing the operator to be exposed to it.
Isolators are defined as sealed enclosures that may contain a qualified controlled environment and are at variance with the surrounding conditions designed and tested to a level of leak tightness to international standards, typically ISO 14644-7 and ISO 10648-2.

Traditional glove-based isolator
Isolators are used for handling products of high potency or toxic hazard. They typically work with operator exposure levels (OEL) below 1 µg/m3. This article, however, does not look at this type of isolator, but suffice to say that these units are typically located in other parts of a facility (e.g. sampling, weighing or dispensing of raw material, sieving, milling, blending, formulation, compounding, granulation, capsule filling, tabletting).
The main causes of injectable drugs product recall are the presence of particles, lack of sterility and bacteria contamination
Today, the requirements for aseptic and containment conditions are increasingly combined either because the product is highly toxic or due to the condition of the sterile product. In these cases, when there is an event or safety breach, the isolator turns into negative pressure to protect the operator.
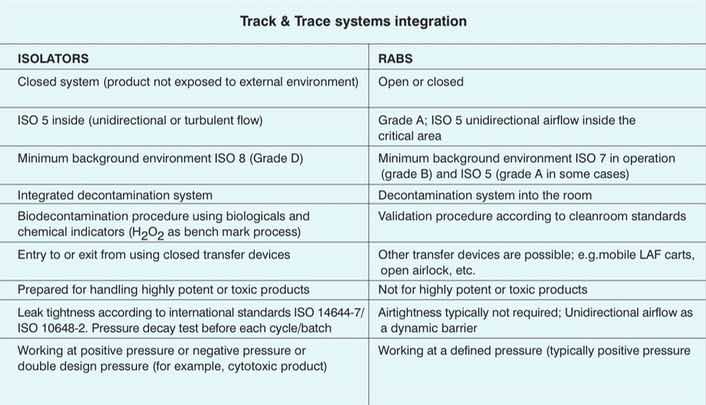
Main differences
The main differences between isolators and RABS are the level of tightness and a different validation system. While validation of an isolator is performed by bioindicators, the validation of a RABS is similar to a traditional cleanroom. Both systems are built in similar ways using similar components, and this sometimes causes confusion in the industry.
The initial investment is, of course, a key element to factor; it is true that isolators or RABS require a higher expense compared with the traditional solution, but it's compensated by considering the savings in operating costs, energy savings —by moving much less air— and space savings. The main differences are presented in the table below.
Track-and-trace systems integration
Beyond the traceability of the product focused on avoiding counterfeit medicines already covered by the regulation of serialisation, track-and-trace systems can be introduced into aseptic processes or related procedures, so as to aid production and maintenance tasks that are difficult to perform for the operator through the gloves.
Some examples of traceability systems applications into aseptic areas include:
- Step-by-step validation (manual or automatic) so that each step of the process is registered and doesn’t allow continuation to the next without having validated the previous one
- This approach drastically reduces the risk of human error. In some special applications, it is possible to video-record the complete process including, the validation of each step through the use of high definition cameras and AI
- Identification systems for components, such as RFID (radio frequency identification) in glove positions to draw the integrity test —and on the glove itself— to plot the number of operations and sterilisations of the glove
- Identification systems for components in direct contact with the product, such as identification of a bottle of liquid disinfectant, a single-use bag (SUS), or identification by RFID of the main components of the product already weighed
- Continuous and instantaneous monitoring of viable and non-viable particles using fluorescence detection optical technology
- Use of monitoring cameras to check the correct performance of inherent manual operations (e.g. filling or weighing of components in the aseptic environment)
- Use of connectors with RFID in pipes to avoid human connection errors. The valve shouldn’t open if the connected pipe is not adequate
- Line identification systems prepared for the next batch; e.g. format changes, sanitisation and decontamination
- Automatic inspection systems, for example, for the detection of vials that don’t have a stopper by mistake or are incorrectly positioned. The vial will then be automatically removed from the line
- Record of any event and registration by means of an audit trail to identify the operator and the exact moment of the intervention
In any case, the monitored results and events recorded over time must be considered and analysed before the final release of the batch or product. The data must be integrated into a database, such as a module of the manufacturing execution system (MES) or a specific database that is connected to the MES.
The bottom line
Traditional aseptic zones where the whole room is a Grade A unidirectional airflow and the operator moves freely inside the room are being replaced by isolators or RABS.
This change of paradigm is mainly due to the fact that these units have the same purpose: to avoid contamination of the product. They achieve that while optimising the energy used in ventilation, reducing space as well as improving product safety by physically separating the operator from the product. Considering that the operator is the main source of contamination, the natural to wonder whether it’s possible to fully automate the process. Many tasks in the aseptic process are completed without the operator; the operator doesn’t add value in the process and doesn't necessarily have to be inside the aseptic zone.

Example of robotic arm for movement of materials
Examples of this shift in the market is the use of robotic arms for movement and/or positioning of containers (e.g. trays, vials, bottles), automatic loading/unloading systems for vials through magnetic levitation systems or the use of RABS or isolators with fully automated gloves where the intervention of the operator during the operation is null.
If full automation is not possible, we must minimise human intervention and always make the decision based on a risk assessment, identifying the hazard and addressing it, applying the proper corrective and preventive actions.
The use of track-and-trace systems to improve the safety of the process is a clear trend and these are being incorporated to improve the safety of both the operator and the product. GMP facilities require the use of available technology to ensure both.
The decision on which technology to use should factor the investment cost, product potential, the flexibility of the line to work with different products or formats, and the possibility of automating the process in the future to improve product safety and quality.
N.B. This article is featured in the June 2019 issue of Cleanroom Technology. The digital edition is available online.




