TTS Pharma, a producer of cannabinoids, including cannabidiol CBD, has released the results of independent analyses of its CBD oil for use in food, cosmetics and nutraceuticals. The company engaged Fera Science, a public-private analytical laboratory, to undertake a series of in-depth analytical tests to ensure that its product fully complies with all regulatory requirements. TTS Pharma also requested analyses of a range of other CBD products currently available on the UK market.
THC and CBN are two psychoactive cannabinoids found in cannabis. The Fera analysis showed that TTS Pharma’s CBD oil is within the THC and CBN limits of detection (<0.0001%) and confirms that the isolate is exempt from the Misuse of Drugs Regulations (UK Government 2001).
“We are committed to creating the purest CBD oils and isolates, setting standards over and above current regulation to raise the bar in CBD product production,” he said.
Speaking at a press conference in London, Mark Tucker, TTS Pharma CEO, explained that as the hemp plant — the source of CBD — naturally absorbs contaminants from soil, water and air (phytoremediation), commercially available CBD may also contain a variety of these harmful impurities.
We are committed to creating the purest CBD oils and isolates, setting standards over and above current regulation
Tucker said the company applies pharmaceutical standards to produce the highest quality, legal, fully licensed cannabidiol (CBD) for use in food, cosmetics and nutraceuticals. To achieve that, TTS Pharma uses the CHOtrak, a fully traceable supply chain system that employs in-line production controls to establish provenance throughout the supply chain.
Market research
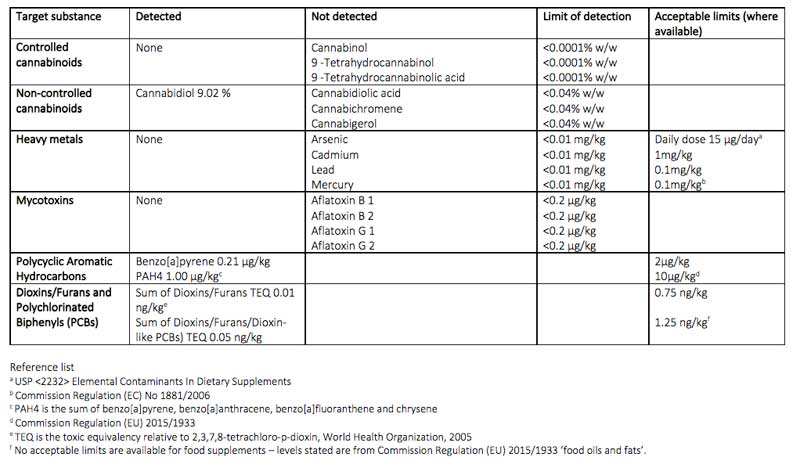
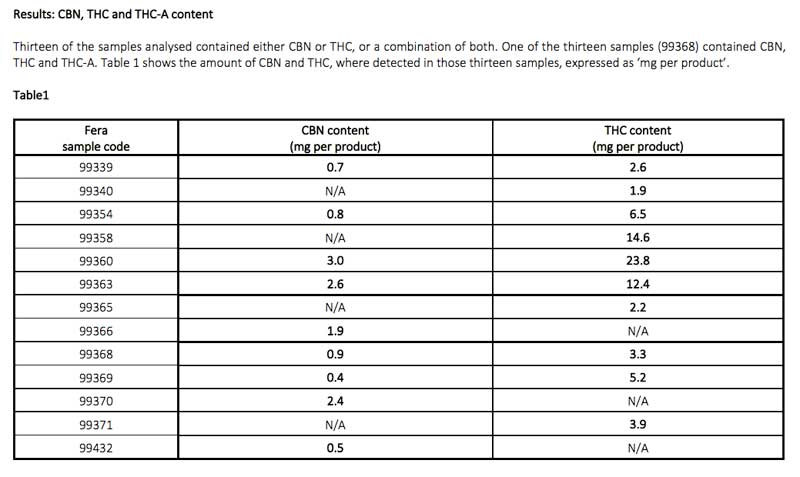
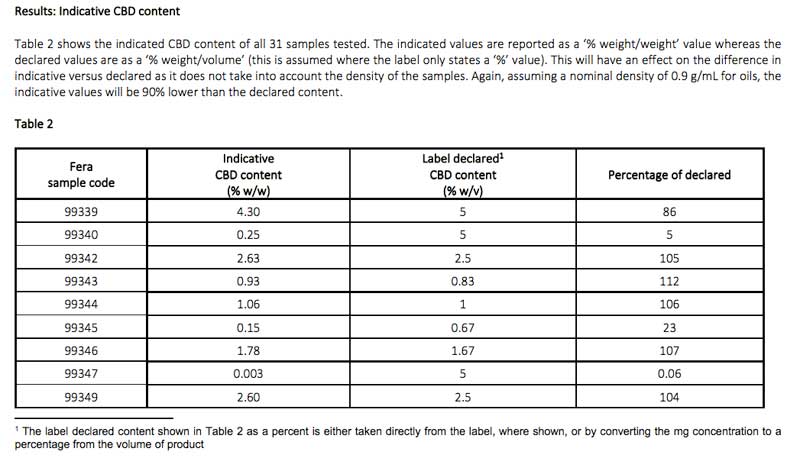
TTS Pharma requested Fera a simultaneous analysis of 31 CBD consumer products currently available on the UK high street, and data revealed products contained illegal levels of THC and CBN and harmful environmental impurities, and inaccurate labelling of CBD content, as seen below in Table 1 and Table 2.
Of the samples tested, the results revealed:
- Inaccurate labelling of CBD content
- CBN and/or THC were found in almost half of the samples (13, 41%), most of which were above the legal limit
- All ten samples found to contain THC were above the 1mg legal limit
- Nine of the thirteen samples contained CBN
- Four of the nine samples which contained CBN were above the 1mg legal limit
- Less than half (thirteen of the samples tested) were within 10% of the stated level of CBD
- Four samples contained less than 50% of the level stated, and five exceeded the level stated
Further analysis of three of the UK’s top-selling CBD products showed presence of some toxic substances: terpenes, heavy metals, polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), polychlorinated dibenzo-p-dioxins (PCDDs/dioxins) and polychlorinated dibenzofurans (PCDFs/furans).
These substances may have significant health implications including cancer, liver and kidney damage and neurological toxicity.
TTS Pharma CBD oil was shown to be free of all of these impurities. However, the three popular available products tested were shown to contain some or all of these impurities.
All three of our independent analyses demonstrate an urgent need for greater regulatory control of currently available CBD products to safeguard consumers
“All three of our independent analyses demonstrate an urgent need for greater regulatory control of currently available CBD products to safeguard consumers from making product choices which ultimately may be risking their health,” Tucker said.
For Tucker, the implications of such a rapidly growing market, being flooded with illegal products, cannot be ignored. “Without a detailed analysis of CBD products, by manufacturers and suppliers, consumers cannot be sure whether the product is safe to consume,” he added.
In light of these results, TTS Pharma claims it is the only UK company able to offer quality assurance from seed to shelf, with detailed product traceability plus product liability insurance as an integral part of its product offering.
