Dr Fabio Di Francesco is a medical cannabis expert and has been working in a compounding pharmacy in Rome, Italy, for two years. His journey started upon completion of a degree in Pharmacy in January 2017 from Sapienza University of Rome and continued with a specialisation course in pharmaceutical compounding. “Immediately, I was interested in medical cannabis and how it is possible to get a pharmaceutical dosage form with cannabis flowers,” he tells me.
We met at an event hosted by a cannabis company in London (UK) in September. In a rather casual yet insightful conversation, he reveals details of his work, the dosage forms of medical cannabis products and his views on the European cannabis market.
Cannabis formulations
Today, Di Francesco works on cannabis formulations for Ponzi SAS, a Rome-based compounding pharmacy, and on the manufacture and development of medical cannabis products. Dutch cannabis supplier Bedrocan is the primary source for the company.
Di Francesco tells me that in 2019, the pharmacy has sourced the majority of its supply from the Netherlands totalling 700 kg/year and that Canadian supplier Aurora is also engaged with 200 kg/year. “In addition, we have Italian Cannabis produced in Florence, but only a few kg/year,” he says.
In Italy, we don’t have cannabis oil supplied by cannabis companies, so the manufacture of dosage forms is the responsibility of compounding pharmacist
Speaking about his day-to-day work, Di Francesco explains: “My job is to manufacture personalised pharmaceutical dosage forms prescribed by a medical doctor (MD). In most cases, these dosage forms are either no longer commercially available, commercially available but not in the desired dosage, or commercially available in an unusable pharmaceutical form, which is common in paediatrics, for example. So, it all comes down to cases where there is a need to personalise the therapy.”

Cannabis flowers are imported for medical formulations
Di Francesco tells me that cannabis is an ingredient of these medicines because there are no other drugs available on the market, except for Sativex among a few others that are dispensable only in hospitals. “Furthermore, in Italy, we don’t have cannabis oil supplied by cannabis companies, so the manufacture of dosage forms is the responsibility of compounding pharmacist,” he says.
Dosage forms
In Italy, the importation of cannabis for medical applications involves flowers or cannabis in granulated form. “I use many machines to manufacture medical cannabis products depending on the concentration that requires each prescription or which pharmaceutical forms the MD wants,” Di Francesco explains.
“MDs and compounding pharmacist usually stay in contact to find out the exact dosages and pharmaceutical forms to manufacture for every specific patient,” he adds.
Di Francesco tells me the dosage forms he works on can vary a great deal. “The MD prescribes the pharmaceutical form that he considers most appropriate for his patient. Then the compounding pharmacist has the formulation and chemical skills to manufacture the different formulations,” he says.
The forms available that is possible to manufacture range from cannabis bags, capsules, resins, eye drops, suppositories, creams, transdermal gels, edibles and syrups.
The MD prescribes the pharmaceutical form that he considers most appropriate for his patient
Di Francesco reveals the most prescribed dosage is Bedrocan 22% THC <1% CBD in olive oil. “With this prescription is it possible to get an oil with 1.5 % THC,” he tells me.
“Decoctions are prescribed a lot, and it is possible to get 5 mg or 20 mg THC depending on the strain used,” he says, making it clear that cannabis is not prescribed for smoking. “The cannabis bags are prescribed for decoction or for vaporising; the only person that chooses how to use the medical cannabis dosage form is the MD,” he points out.
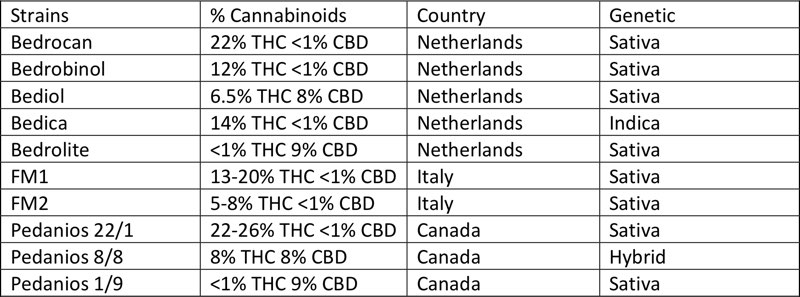
Strains in use for medical cannabis currently available in Italy
Contamination control
Speaking about the laboratory equipment, he says: “Vertical laminar flow hoods [are used] for making eye drops, because this preparation is the only one that requires sterility. For the rest of the products that I manufacture, I use exhaust hood.”
Sterility, Di Francesco tells me, is a critical requirement in the compounding process, and that the lab he works at is an aseptic cleanroom with different graded areas, from A to D.
“When sterility is not necessary you can work without [laminar flow hood],” he points out, noting that he works following the Italian Pharmacopeia, which requires that the area to carry work with powders is separated with other areas to reduce contamination risks. FFP3 masks, nitrile gloves, shirts, cap and shoe covers are all required to prevent contamination.
For example, oil with 15 mg/ml THC, you can get a variation of 1.5 and you can have oil with 16.5 mg/ml or 13,5 mg/ml
Commenting on the contamination control and quality control procedures, Di Francesco points to the German Pharmacopeia where cannabis is present. “Quality-control assessment can include theoretical weight compared with actual weight, apparent pH, specific gravity, active drug assay, colour, texture–surface, texture–spatula spread, appearance, feel, rheological properties, and physical observations,” he explains.
Plus, if an MD wants a standardise oil or resin (extracts) to manufacture, a variation of +/- 10% is allowed. He explains: “For example, oil with 15 mg/ml THC, you can get a variation of 1,5 and you can have oil with 16,5 mg/ml or 13,5 mg/ml.”
Quick-fire questions
What do you know about other markets?In Europe, the cannabis market is growing fast. Germany is probably the country with a medical cannabis system similar to Italy, and in some aspects even better. In the UK, medical cannabis has been legal since 2018, but the system is far behind and to have a prescription, it is necessary to spend a lot of money in private clinics. Then, there are countries where in addition to being legal, medical cannabis is also accepted for recreational purposes, like the Netherlands and Spain. Currently, no other European country has legalised cannabis.
How do you know what illness can be treated with medical cannabis?
Currently, everyone that talks about cannabis end up saying “more research is needed”. This statement is only partially true because it is possible to search on PubMed and see how many studies we have today. Cannabis is prescribed for many diseases, and most prescriptions are commons to pain, epilepsy, insomnia, glaucoma, Tourette syndrome, nausea and vomiting in chemotherapy patients, etc.

Dosage forms range from cannabis bags, capsules, resins, eye drops, suppositories, creams, transdermal gels, edibles and syrups
What is the feedback of patients?
Most patients have benefits with the treatment and have a complete absence of side effects. Many times they leave the treatment with cannabis not because of the side effects, but because they cannot afford the cost of the therapy.
For example, epilepsy patients must pay medicinal cannabis because it is a disease that the Italian health system does not recognise. GMP-grade CBD in high dosages is expensive.
Pharmaceutical cannabis market
Di Francesco tells me that the Italian health system recognises many diseases and that the patient pays only a small fee, or in some cases nothing. “However, other diseases are not recognised, and the patient must pay the full price. For example, pain is a recognised disease and epilepsy not,” he says.
A general medical doctor or specialist can prescribe cannabis in Italy. Di Francesco tells me that even the veterinarian or dentist can prescribe cannabis. “The patient must simply go to the doctor to get the prescription, and then the patient takes it to the pharmacist in a compounding pharmacy. The patient will be advised to collect the cannabis, and whether to pay or not, depending on the disease it is for.
Many MDs are prescribing medicinal cannabis partly due to proper training and education on the benefits that cannabis has to the many diseases in patients
Medical cannabis has been legal in Italy since 2007. Di Francesco explains that initially, doctors who prescribed cannabis were very few, but that has changed. “Many MDs are prescribing medicinal cannabis partly due to proper training and education on the benefits that cannabis has to the many diseases in patients,” he says.
Commenting on what is challenging the development of the medical cannabis market, Di Francesco says: “The future should ensure that there are agreements in place, so medical cannabis products are free for patients and ensure that they have access to it. Currently, importations from the Netherlands and Canada are not sufficient to guarantee therapeutic continuity for patients.”
The future should ensure that there are agreements in place, so medical cannabis products are free for patients and ensure that they have access to it
Availability of cannabis is a big issue in the market. According to Di Francesco, even the Italian production, carried out by the Military Pharmaceutical Chemical Institute of Florence, does not produce sufficient quantities for Italian patients.
“Most of the time, when the patients can’t get medicinal cannabis in the pharmacy because it is not available, they buy it on the black market. Imagine a mother of epilepsy child that can’t get the medicine for her baby. What should she do?” he concludes.
