The interest in advanced therapy medicinal products (ATPMs) – or CGTs (cell and gene therapies) – for human use continues to grow due to the promising therapeutic potential of these drugs. Never have approvals of such products from the European Medicine Agency (EMA) or the US Food and Drug Administration (FDA) been so popular as in 2017 and 2018. If we look at clinical trials currently running in the US many treatments involving ATMPs are at an advanced stage. Consequently, the increasing popularity is generating a growing demand, which will suddenly lead to requirements for mass production of these biological products.
ATMPs offer groundbreaking opportunities for the treatment of disease and injury. These products are classified into three main areas: gene, which work by inserting/substituting recombinant genes into the body to treat genetic disorders or any other gene-linked diseases; somatic-cell, which contain cells or tissues that have been manipulated to change their biological characteristics to cure, diagnose or prevent diseases; and tissue-engineered, modified to repair, regenerate or replace degenerated or injured human tissue.
These advanced therapies are subject to legislation and must comply with current good manufacturing practices (cGMP), Annex 1 and 2, to allow commercialisation; production is assimilated to traditional sterile pharmaceutical practices.
Today, there are different manufacturing scenarios. When we deal with the treatment of rare diseases, the quantities of cell cultures required are limited. On the other hand, when considering the cell therapy products needed for diseases such as cancer, or diseases that are spread across a large population, the quantity of cells required is incredibly high, thus more and bigger cell factories are necessary.
The current process for developing ATMPs usually starts from a laboratory approach, where the initial formulation and doses of the new drug are set up and tuned. It is not uncommon to find that all the preclinical and Phase I clinical studies run according to protocols prepared in this way.
A laboratory approach is different from the principles set in cGMP and Annex regulations. However, there has been success in single procedures for the manufacture of these cell products. But the reality when we face the transition from an individual culture system to mass production, is far from simple: it requires additional classified spaces, process scalability (to increase production capacity without losing quality), extra attention to integrity and cross-contamination issues (when facing multi-patient jobs), and an overall quality outlook that needs to be introduced and carefully evaluated.
To increase manufacturing capacity, production facilities must either expand, or production must be faster wherever possible by speeding up some processes with automation or parallelising activities that can be run simultaneously.
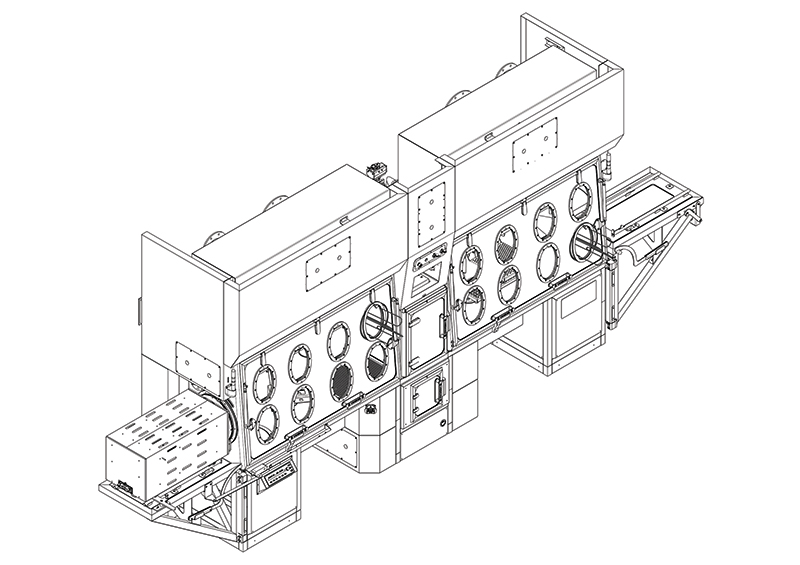
Modular incubation system FlexyCult coupled with isolator technology
Expanding a cleanroom facility is a costly and challenging task; there are considerations about the footprint, connections with the existing facilities, the time necessary for expansion and loss of continuity in production; it also implies arduous logistical considerations in managing construction work inside an environment that should be aseptic.
An alternative proposal
We believe that some of the issues mentioned above can be addressed by moving from an “all-in-one room” solution, typical of the current cleanroom approach, to an integrated modular system, benefitting from isolation technology advantages and capabilities offered by sterile connections.
Following these guidelines, we have designed and built a Grade A modular incubation system that we call FlexyCult. It is a Grade A incubator (a HEPA-filtered closed air loop system kept in overpressure) with all the characteristics of a laboratory incubator: temperature, relative humidity and carbon dioxide are controlled and maintained within given boundaries.
The innovation is that the incubator itself is stowed in a docking station where all the utilities are concentrated and shared with various incubations modules in a 3, 6 or 9 module configuration. Single incubators can then couple with an isolator (see drawing) through a dedicated rapid transfer port (RTP) – ie an aseptic connection. Cells undergoing cultivation are then transferred to the isolator and processed according to the procedural needs. The isolator is equipped with all the necessary laboratory tools for cell processing and can be personalised to a customer’s requirements. The equipment is a closed system and can be located in a Grade D area, thus meeting regulations.
The proposed approach meets expectations of the cell culture environment for several reasons: the sterility is superior due to the segregated environments in which the cells are manipulated; the cells are also entirely separated from the operator; cells are never exposed to direct contact with humans (recognised as the main source of contamination); cells are always manipulated under Grade A conditions.
Vapourised hydrogen peroxide (H2O2) sterilisation can be carried out in the incubator when it is empty, as well as in the isolator when not in use between different production cycles.
The footprint of the FlexyCult system (incubators and isolator) is smaller than that of a Class B cleanroom required to perform comparable activities.
The energy requirement of running the same procedure in a cleanroom is lower, and so is the volume of air exchanged. Consequently, the operational costs for running the same facility capacity, in terms of power consumption and environmental monitoring requirements, are much lower; savings are estimated at around 60% of the yearly costs of the standard cleanroom approach. Operators can also work without special sterile gowning, saving costs in disposable wearables and time for gowning/de-gowning, which definitely translates into a productivity gain.

RTP connected and open access to incubator
Proven application
The Comecer approach to cell culture applications has been chosen by CO.DON AG, a German biotech company that already produces well-established autologous cell therapy for tissue regeneration. The procedure enables the regeneration of high-quality, biomechanical load-bearing and pressure-resistant cartilage tissue. By using the potential of autologous cells, joint cartilage defects can be treated successfully. The three-dimensional structure of autologous cultured chondrocytes and autologous self-constructed matrices leads to the reconstruction of cartilage-specific functional properties.
The procedure has already been successful in more than 12,000 patients. The product was approved by the Committee for Advanced Therapies (CAT) of the EMA in July 2017 for commercialisation within the European Union and the company has started a project for improving its production capacity, with the intent to reach approximatively 4,500 manufactured batches per year. To accomplish these goals a total of 16 docking stations – each composed of 9 FlexyCult incubators, 3 FlexyCult isolators and accompanying H2O2 sterilisation stations – have been arranged to fit into a brand new dedicated laboratory (see photo on p17). Isolators are managed by operators in a Class D environment while incubators can be located in a non-classified environment. Extensive use of software controls allows easy management of incubator retrieval from the docking system and automatic guided vehicles run into the lab transporting appropriate elements according to operator instructions.
With this level of automation, operators can fully concentrate on the critical operations, minimising their involvement in tasks not strictly linked to their specific competency.
Controls, cross-checks, tracking and logging of the operations in each incubator are controlled via software and barcode and radio-frequency identification (RFID) technology is utilised. These tools and its design make the system robust, easy to use, to the highest regulatory standards.
Respect for quality requirements is a key point in any drug production process. Robust manufacturing makes the production independent from interruptions and ensures consistency of the result over time.
When dealing with ATMPs, living cells used in these products must be viable, pure and, most importantly, sterile. Containment of the product is crucial as the process is patient specific and involves highly qualified operators.
Very often we do not have a second manufacturing chance, so requirements are even more stringent. Larger quantities need to be produced without compromising on quality and the price needs to be feasible so that healthcare organisations can afford the treatments.
The proposal of a modular, partially-automated manufacturing system for ATMPs based on isolation technology makes possible its use to reduce the production costs of drugs. Moreover, the system is designed to be expandable, allowing extensions of the production in successive steps.
Comecer continues to address the remaining challenges and hopes to improve the process of introducing raw and starting materials, and increase the use of automation in the system to always improve standards.
Editor's note: This article is featured in the July issue of Cleanroom Technology. The digital edition is available online.
