Applying to all biocidal products, EU regulation 528/2012 Biocidal Products Regulation (BPR) came into force in September 2013. The regulation is designed to control the selling or placing on the market of biocidal products. It involves the analysis of a biocidal product’s efficacy, toxicity, environmental fate and risk during use. This article aims to summarise some of the key points of concern when sourcing BPR-approved products.
The BPR is a two-step process. Firstly, biocidal active ingredients must be authorised as Actives, and an important factor in the Active assessment is how a biocidal product is intended to be used. The Active biocidal product must be authorised for use under specific categories, known as Product Types (PTs). There are 22 different PTs ranging from PT1 “Human Hygiene” through to PT22 “Embalming Fluids”.
To be marketed for a specific application, biocidal products must be authorised for use within a specific PT. For example, vapourised hydrogen peroxide (VHP) used within cage racks in an animal research facility would require PT3 “Veterinary” authorisation.
Biocidal products often possess authorisations for several Product Types. Users should always ensure that a disinfectant product or system is authorised for their specific use
If a biocidal product is authorised solely for use in PT1 “Human Hygiene” applications, it cannot be used as a disinfectant for hospital surfaces, which requires a PT2 “Public Area” authorisation. Biocidal products often possess authorisations for several PTs (or use areas). Therefore, users should always ensure that a disinfectant product or system is authorised for their specific use.
Efficacy and proving performance
Next is the second step. Once an Active has been authorised, manufacturers of biocidal products and systems using or containing that Active are required to submit a technical dossier to the European Competent Authority (CA) by a deadline.
The dossier should include evidence for supporting the use, risk, toxicity and efficacy claims, and must also indicate the process parameters used to support such claims, including the application equipment used and contact times, where appropriate. In other words, compliance with the BPR requires that both the biocide (in this case the hydrogen peroxide aqueous solution) and the equipment (in this case the vapour generator or nebulising/aerosolising machine) are tested together to support any efficacy claims. In the case of hydrogen peroxide, the deadline for technical dossier submission was February 2017.
Compliance with the BPR requires that both the biocide and the equipment are tested together to support any efficacy claims
If a manufacturer of a biocidal product or system does not submit their dossier in time, it is an unauthorised biocidal and illegal to market.
The role of Article 95 in the BPR
Article 95 of the BPR came into force in September 2015. It states that a supplier of any Active intended for use within a biocidal product or system must be registered under the BPR process.
The supplier of the Active must be present on “The Article 95 List”. Producers of disinfectant products or systems may only use Actives sourced from Article 95-compliant suppliers (i.e. they are on the list), and may only market their product for a specific use if that use or Product Type is associated with the Active on the Article 95 list. Products marketed as disinfectants or biocides utilising biocidal Actives from a non-Article 95 compliant source are illegal.
In practice, several hydrogen peroxide-based decontamination systems can utilise hydrogen peroxide sourced from many different manufacturers or sources. An example of a commonly used supply is Merck’s industrial hydrogen peroxide. Here, the manufacturer places its industrial hydrogen peroxide on the market for use as an industrial product, with no intention for it to be used as part of a biocidal process.
If a company chooses to use such hydrogen peroxide for a disinfection purpose, then the product is considered to be a biocide, and as the Active does not come from an Article 95-compliant source, it is an illegal use.
Companies using industrial hydrogen peroxide as biocides should review their regulatory position and assess the risk to their business. Users should also be aware that a biocidal product claiming compliance with the BPR, based on the biocidal product containing an Active from an Article 95-compliant supplier, may not necessarily remain compliant with the BPR in the future. Compliance with Article 95 is only a first step to a fully compliant product or system.
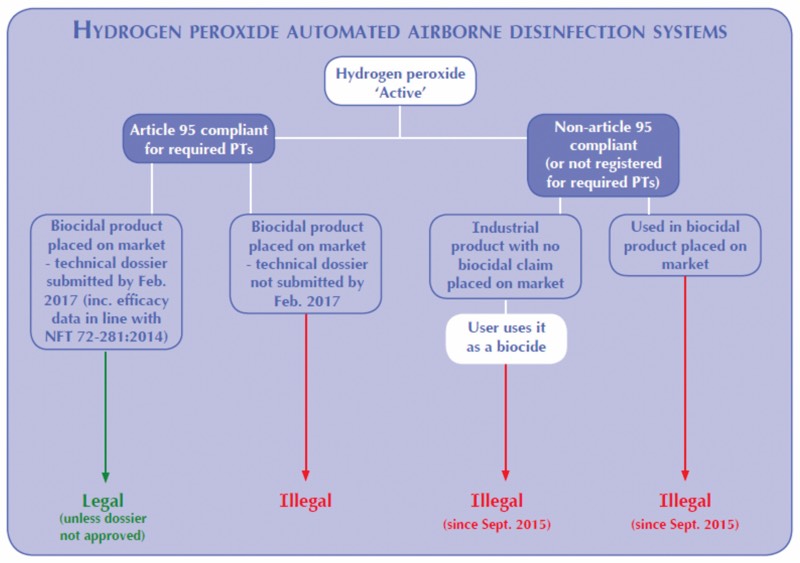
Hydrogen Peroxide Automated Airborne Disinfection Systems
Biocidal products, used with their delivery system, must also undergo authorisation by one or more European CA, which involves assessment of use, risk, toxicity and efficacy. For manufacturers of decontamination systems that deliver biocides via an airborne route, regulators require efficacy data produced using the standard NF T 72-281 (2014). In future, the new European standard EN 17272 will replace it. Disinfectant manufacturers and users can refer to EN 14885 for guidance on various EN standard test methods.
The role of NF T 72-281 (2014)
Biodecontamination systems that distribute a biocide via an automated spray, mist, fog, vapour, or other technique, are considered “airborne automated disinfection systems”. Here, the Active biocidal product (in the case of hydrogen peroxide-based systems, the hydrogen peroxide liquid) must be tested in combination with its application method.
For example, a disinfectant tested and authorised for nebulisation, could not be used with a vapourisation-based system and vice versa. The application processes are different, and thus the efficacy data is not applicable to another dissimilar.
The European Chemicals Agency has produced a detailed guidance document on the efficacy assessment requirements for biocidal products, particularly in PTs 1-5. Airborne disinfection systems must be tested against NF T 72-281 (2014) under the PT use scenarios claimed by the biocidal product.
NF T 72-281 is a challenging test against a wide range of microbiological organisms (bacteria, viruses, fungi, yeasts, spores, mycobacteria and bacteriophage) with soiling conditions relevant to the claimed use scenario. For example, a hydrogen peroxide-based system intended to be used in a hospital without any qualifications must pass the Human Health section of the test, which stipulates:
- 5-log reduction of the specified bacterial strains (such as Pseudomonas aeruginosa)
- 4-log reduction of yeasts and fungi
- 3-log reduction of spores
- 4-log reduction of viruses and
- 4-log reduction of mycobacterium
The NF T 72-281 test method attempts to assess real-world application of the biocide via its associated delivery system or generator. Organisms are dried onto stainless steel tokens on the opposite side of the room to the generator or equipment, facing away from it. The biocidal liquid must be tested in combination with the specified manufacturer’s generator. The dose of hydrogen peroxide and its contact time required to achieve the stipulated reductions should be described.
The new EN 17272 is expected to increase the testing requirements on automated airborne disinfection systems. Preliminary drafts include the need to conduct a distribution test and also incorporate testing that acknowledges the implications of the SA:V ratio in airborne disinfection-based decontamination, requiring tests for both large (> 30 m3) and small (< 4 m3) enclosures. Isolator manufacturers with on-board and in-built airborne disinfection systems should be aware of and understand the implications of this new standard.

Users of all airborne disinfection systems and facilities looking to purchase such systems should question manufacturers as to whether their systems have been tested to, and passed, the NF T 72-281 (2014). Pictured is Bioquell ProteQ Mobile Room Biodecontamination unit
Implications for buyers
If the NF T 72-281 (2014) test has been passed, users should request the following information:
- The concentration of the biocide used in the test, often given as a percentage, e.g. 35% hydrogen peroxide
- The contact time to kill the organisms specified, usually given in hrs and mins
- The organisms killed (i.e. Aspergillus brasiliensis)
- The level of kill achieved (e.g. >log 5 reduction)
These figures can then be compared with other suppliers who have also passed the test.
All airborne disinfection system suppliers should be working to provide a validated and approved biocidal product under the BPR. If suppliers are planning to meet the regulations by providing a “new” biocidal product to replace an existing one, or supply a new product derived from a biocidal Active sourced, from a new supplier, then biocidal and biodecontamination processes currently conducted by users may need to be revalidated.
Should a new biocidal product be introduced as a replacement, there could be a bottleneck delay in the user business, as all the relevant standard operating procedures (SOPs) may need to be rewritten en masse.
N.B. This article is featured in the October 2019 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.

