All API production environments require strict contamination controls but pharma labs require even more contamination awareness, argues Sean Codling, managing director, CTS.
It is a fact that smaller quantities of today’s new potent drugs can have greater pharmacological effects than their predecessors. Because of this, pharmaceutical production environments rely heavily on strict engineering controls and procedures to minimise exposure risk to operators and provide safe working environments for the large-scale handling of active pharmaceutical ingredients (APIs).
Ensuring exposure safety in the laboratory environment, however, presents different challenges. The laboratory environment will, of course, handle APIs in much smaller quantities than the production environment, but this does not mean that there is less exposure risk to the analyst working with the drug compounds. The analyst can carry out a number of different tasks with the drug and, as a consequence, will be in close proximity to it and at increased risk of inhaling airborne contaminants.
Advancements in nanotechnology and the development of drugs for inhalation have made micronised drugs more common. Smaller particle sizes present greater drug surface area for reaction and are easier to absorb or inhale; this is an important patient advantage, but also something of an increased risk to the analyst. The amount of a drug that will have an adverse pharmacological effect on the analyst can be so small that it cannot be seen.
It is essential that APIs categorised as a potent compound are controlled to minimise airborne concentrations and comply with an Occupational Exposure Limit (OEL) ranging from 30ng–10µg/m³. At this level the drug simply cannot be seen; in fact, an airborne concentration over 100 times greater, at 1mg/m³, will not be easily visible. Particulates that do not remain airborne will settle on equipment and work surfaces; generally these will be larger particles of 50+ µm in size, which would not necessarily pose an inhalation hazard if left undisturbed but will be a touch contamination hazard.
Fifty microns is about half the diameter of a human hair and so still very difficult to see, particularly on light work surfaces common in laboratories. Hygienists and safety managers have a challenge in that they need to put in place exposure control methods for a hazard that is invisible.
Dealing with an invisible hazard requires good engineering control, good design and good training.
More and more equipment is being used to test or manipulate drug actives in some way. Weighing is an obvious candidate, due to the close proximity of the user to the operation. However, there are many other processes that may require containment control, such as particle size analysis, Karl Fischer, micronisation, dispensing, X-ray diffraction, rotary evaporation etc.
Where possible, an off-the-shelf solution is best. With ever more local exhaust devices being added to the laboratory, heating, ventilation and air conditioning (HVAC) control is becoming more challenging, as extract air from the laboratory has to be balanced with fresh, conditioned air from outside, without adversely affecting room pressures and fume hood performance. Containment devices need to be integrated into the laboratory in a way that does not affect the air handling within the laboratory or that of any other containment device.

Laboratory worker using a balance
Containment design
Putting an instrument into a standard fume hood will not always work. Analysts need to be able to work effectively and, wherever possible, with minimum restriction. Fume hoods will not always provide the best environment for sensitive analytical equipment and in addition are not designed for enclosing instrumentation. A good containment device should be ergonomically designed such that the analyst can work comfortably and access as many areas of the equipment as required, without restriction. There are some basic design criteria that all laboratory containment devices should possess, as a minimum standard:
- Air flow alarm to indicate safe or unsafe operation
- Correct filtration for the job that is tested on site to prove efficiency
- Integral base to ensure contamination and samples stay within the footprint of the device
- Recognised flow performance that is tested on site prior to use.
Understanding the equipment and processes that analysts have to carry out in the lab is key to good design, as well as understanding that containing these processes adds a restriction to the way they carry out this work.
If the containment device is too restrictive then the process will take longer, require modification or cause the analyst to work in an uncomfortable way, leading to sample handling errors or other health concerns relating to posture. Devices that are poorly designed will not be used in their “safe mode”. Access doors will be left open, alarms will be disabled, extract and filtration modules will be turned off. This leaves the hygienist and safety managers with a situation that is worse than before.
A safety containment device not being used correctly is worse than having no containment system at all, as it will not be providing a recognised safety factor, while the operator assumes an exposure safety factor that simply is not there.
Enclosure design also needs to take into account service and maintenance requirements to ensure equipment can be accessed easily by the engineer. In many cases, the equipment and surfaces within the containment device will be heavily contaminated; where normal use will provide a safe environment for the analyst, the tasks that the engineer may need to carry out could mean reaching into the containment device and coming into direct contact with contaminated surfaces.

Benchtop X-ray diffractometer
The very fact that a process is contained will mean that the containment device is, in effect, a hazardous zone and that contamination will be concentrated in this area. Therefore, there is a great deal of emphasis on the user to handle the device correctly and understand where and how contamination occurs. Designing and implementing a containment solution will, of course, provide a safety factor, but the real safety control comes from the way in which the analyst uses the device.
Contamination awareness training is an effective way to help the analyst understand the reasons why certain processes are contained. Key areas to understand are:
- Awareness of potent compounds
- Exposure routes – how will the drug get into the blood stream?
- How does contamination move about the laboratory?
- How can contamination be minimised?
In many cases, analysts will work on more than one drug, while within the same laboratory their colleagues may be handling other drugs at various potency levels. In the event that an analyst has adverse reactions due to exposure, it could be difficult to establish whether it was due to the drug they were handling, or that being handled by a colleague.
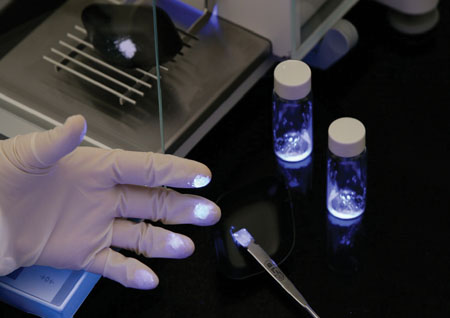
Contamination spots highlighted using UV
An ideal objective to reach would be that there would be clear visible handling techniques common throughout a particular laboratory, so that it is clear for new analysts working in the lab that good sample handling techniques are part of their routine.
Hands-on training in the laboratory can assist in developing safe handling SOPs, through shared knowledge of how contamination occurs and by demonstrating proven sample handling and cleaning techniques.
Analysts need to be aware of contamination in the laboratory, how contamination collects on equipment and surfaces, and how it can be transferred. This can be easily achieved using specialised UV detection equipment to monitor contamination zones and to ensure equipment and surfaces are clean both for analysts’ safety and also for external service personnel carrying out repair and calibration work. By identifying contamination hot spots and monitoring these, analysts can develop SOPs to ensure exposure is minimised and surface contamination on instruments and enclosures is minimised.
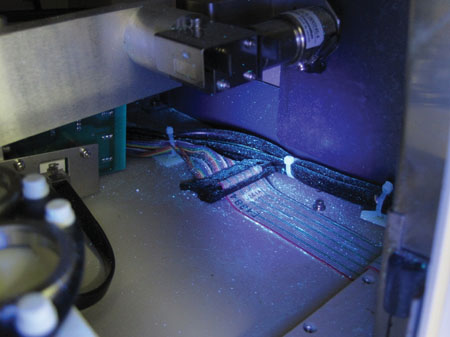
UV lighting used to identify contamination inside the X-ray diffractometer system
Containment Technology Services (CTS) specialises in the design, construction and commissioning of mobile and bench-mounted containment systems in the pharmaceutical industry. The company also offers contamination control and safe handling workshops, based on-site in the customer’s lab, designed to provide the analyst with the knowledge and handling techniques important in implementing an effective containment solution for their particular process.
Effective engineering safety controls along with good awareness training will allow analysts to work safely as they will understand the risks associated with handling active drugs, and ways in which personnel can become exposed. They will have the techniques to implement SOPs to ensure exposure risk is minimised.




