A cleanroom is only ever as clean as the materials taken in and the processes carried out in the room. As cleaning materials can affect that cleanliness, Dr Tim Sandle describes a recent study into particle generation from non-cleanroom and cleanroom wipes.
Cleanroom wipes are used to clean different kinds of surfaces. The essence of using wipes to clean the surfaces and equipment is to ensure that any bacteria or fungi present as contamination on the surface are removed and, where the wipe contains a disinfectant, for the majority of the micro-organisms to be killed.
There are many different types of cleanroom wipes. Some are dry wipes and others are pre-saturated with a disinfectant (used to clean-up spills or to absorb liquids and to disinfect the surface1). With saturated wipes there are two important considerations: the efficacy of the disinfectant and the particle generation from the use of the wipe. Neither of these considerations has been examined in any great detail, based on the limited number of published studies.
In terms of disinfectant efficacy, there have been studies on the disinfectants used to saturate the wipes but little examination of how effective the disinfectant is once it is bound into the wipe fabric and used to wipe a surface (the only standard for such an examination is applicable to the food industry).
There have been studies on the disinfectants used to saturate the wipes but little examination of how effective the disinfectant is once it is bound into the wipe fabric and used to wipe a surface
This article is concerned with the second consideration: the generation of particles when wipes are used in cleanrooms.2 Although some types of wipes are prepared under clean conditions (i.e. with the alcohol mixed with Water-for-Injections or deionised water and each pack of wipes being double bagged and sterilised by irradiation) this, in itself, is not an indication of cleanroom suitability. While irradiation – provided the cycle has been properly validated – will destroy any bacterial contamination the verification of a cleanroom’s cleanliness is through the measurement of airborne particles.
Based on the materials of manufacture, all wipes will generate a certain level of particles.3 This article centres on a study conducted on three cleanroom wipes: two designed for use in ISO Class 5/EU GMP Grade A cleanrooms and one designed for use in hospitals and dental practices.
Cleanroom wipes are not classified as a medical device and there are no regulatory standards that apply to their manufacture. Cleanroom wipes purchased from reputable suppliers are made from low particle shedding materials, are packaged in cleanrooms and, where necessary for the highest classes of cleanroom, are sterilised using gamma irradiation or ethylene oxide.4
The generation of airborne particles within cleanrooms is something that must be controlled. Cleanrooms are classified based upon the concentration of airborne particles within a given volume of air. Once classified, most cleanrooms are monitored on a regular basis for particles, as part of the cleanroom user’s environmental monitoring regimes.5 Within pharmaceutical clean-rooms, this is for particles ranging in size from 0.5µm and larger (with an additional requirement for measuring particles of a size 5.0µm and larger for EU and WHO graded cleanrooms).6
Particles in cleanrooms can be generated from a number of sources, such as people, equipment and fibres. Cleanroom wipes are a potential source of particulate contamination. With the assessment of cleanroom wipes general cleanroom standards are in place for assessing particle generation under controlled conditions, such as those produced by the IEST7 or ASTM8. The methods applied include the Helmke drum test method, which is commonly used to test cleanroom garments.9 This method is used to quantify particles dislodged through the application of mechanical energy under dry conditions as a means of simulating particle shedding from the surface of the fabric during use.
Cleanroom wipes are not classified as a medical device and there are no regulatory standards that apply to their manufacture
Materials being tested are tumbled in a rotating drum to release particles from the fabric in a controlled manner, while a discrete-particle counter is used to sample the air within the drum.10 Alternative methods11 to the drum test include: vacuuming particles from standardised portions of material 0.8µm membrane filter and microscopically counting and characterising particles; using a particle containment dispersal test chamber (body box), which is expensive and only really applicable to garments; liquid extraction using solvents and filtering to examine the extractables from the treated material; and, for the electronics industry, conducting electric charge and static decay measurements.12–14
These methods, however, do not simulate the actual activity of moving the wipe along the surface and do not test wipes within pharmaceutical grade cleanrooms. The ASTM 51 standard itself notes the problem of correlating results from the drum test with actual cleanroom application: “no direct correlation has been established between test methods and their relationship to air cleanliness, and none is implied by this document”.
To address concerns with the repeatability and relevance of the drum test, a study was conducted in an independent cleanroom to assess cleanroom wipes under practical conditions.
Methodology
The study was conducted on three types of cleanroom wipe coded A, B & C. (A) was a schülke wipe called Perform, and (B) an un-named competitor’s wipe, both of which are manufactured to be used with ISO Class 5/EU GMP Grade A cleanrooms and both of which were saturated with 60–70% propan-2-ol. A third type of wipe, (C), used for healthcare instrument and device cleaning (i.e. not a cleanroom wipe) was saturated with 35% propan-1-ol and 25% ethanol. (Given the similar molecular weights, the alcohol type would not have a significant impact on the study, which is focused on wipe grade).
Each of the wipes was manufactured from a mix of polyester and cellulose and packaged in sachets (or pouches), which permitted them to be removed individually.
The study was conducted in a clean environment (an isolator operating at ISO Class 5) so that the background level of particles was minimised (<10 particles of ≥0.5µm). The isolator contained a strip of 304 grade stainless steel, for the wiping activities, which had been subjected to a hydrogen peroxide vapour decontamination cycle. The particles were measured using an optical particle counter.
Different conditions were measured, with the aim of simulating different steps in relation to using wipes within cleanrooms:
- a) Opening a packet of wipes;
- b) Removing wipes from the packet;
- c) A slow wiping technique;
- d) A fast wiping technique.
The slow wiping and fast wiping techniques were designed to see what happens when stresses and frictional energy are generated. For these two steps five back-and-forth actions were undertaken either slowly or quickly (these were qualitatively assessed, with the “fast wipe” being the pace that would normally be applied to a wipe within a cleanroom, as assessed by experienced cleanroom operators).
| Table 1: EU GMP Grade A particle levels | ||
| EU GMP | 0.5µm (counts per cubic metre) | 5.0µm (counts per cubic metre) |
| Grade A | ||
To demonstrate reproducibility, 20 packets of wipes were opened for each of the three brands; a wipe from each packet was removed and the airborne particles measured. Following this, the slow and fast wiping techniques were measured for each wipe. EU GMP Grade A particle levels15, which are the most stringent (see Table 1), were used for the evaluation.
The results of the study showed that the levels of airborne particles increased through each step, starting off lower when a packet of wipes was opened; then increasing as each wipe was removed; and then becoming more concentrated for the slow wiping technique; and then reaching the highest level for the fast wiping technique. This was the same trend across all three wipe brands, although far higher particle counts were recorded for wipes coded (C).
When the particle counts were examined against EU GMP Grade A limits, wipes coded (A) and (B) were within the limits, whereas wipes coded (C) were outside of the limits and generated a high level of particles that would be unsuitable for a cleanroom. These results are summarised in Figure 1 (for particle count sizes of ≥0.5µm) and Figure 2 (for particle counts ≥5.0µm) for each of the steps.
In addition to the graphical analysis, the results were examined using Student’s t-test for significance. The results indicated that the cleanroom grade wipes, such as schülke’s Perform, produced significantly lower particle counts for each step compared with the lower grade wipes.
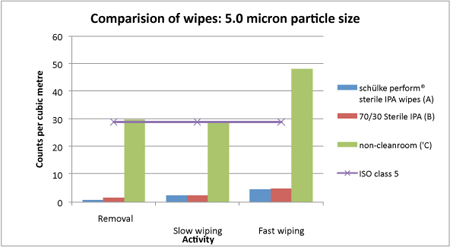
Figure 2: Again, wipes (A) and (B) performed better in all the tests
Evaluation
While no study can simulate every type of cleanroom due to the many variables – the room size, the number of people in it, other contributors to contamination, and the airflow direction and velocity – the proportion of particles present on a surface, and the extent to which these are released,16 and the characteristics of substrates linting from the material17 are important variables. This study is the closest approximation to what happens within the cleanroom environment when a wipe is used.
The results were clear cut: wipes designed for the cleanest conditions were appropriate for those conditions, whereas wipes not designed for ISO Class 5 environments were unsuitable to be used in the cleanest areas.
This article has outlined the importance of particle generation within cleanrooms and has highlighted the difficulties in constructing tests that can assess the particle generation from cleanroom wipes.
The article has summarised the results of a study that examined particle generation from wipes under practical conditions for different steps: opening packets of wipes, removing wipes, slow wiping and fast wiping techniques. It demonstrated that the wipes made from the appropriate materials, prepared under cleanroom conditions and sterilised, such as schülke’s Perform range, are suitable for the cleanest conditions (particularly ISO Class 5 environments), whereas wipes prepared under less stringent conditions and marketed for a lower class of cleanroom should be restricted to such areas due to the high numbers of particles generated.
References
1. J.M. Oathout, Journal of the IES, 42 (3), 1999, 17–26
2. W.K. Kwok and J.T. Summers, J. of the IES, 33 (6), 1990, 33–38.
3. Oathout, J.M. and C.F Mattina, J. of the IES, 38 (1), 1995, 41–51
4. D.W. Cooper, J. of the IES, 39 (3), 1996, 31–36
5. T. Sandle, “Environmental Monitoring” in M.R. Saghee, T. Sandle and E.C. Tidswell, (Eds.): Microbiology and Sterility Assurance in Pharmaceuticals and Medical Devices, New Delhi: Business Horizons, 2011, 293–326
6. T. Sandle, Pharmaceutical Manufacturing and Packaging Sourcer, Spring 2003, 8–11
7. IEST-RP-CC004.3. (2004). Evaluating Wiping Materials Used in Cleanroom and Other Controlled Environments. IEST, Rolling Meadows, IL
8. ASTM E1560 – 06 Standard Test Method for Gravimetric Determination of Nonvolatile Residue From Cleanroom Wipers, American Society for Testing and Materials, West Conshohocken, PA, USA.
9. Recommended Practice (IES-RP-CC003.2) “Garment System Considerations for Cleanrooms and Other Controlled Environments.”
10. D.S. Ensor, J.M. Elion and J. Eudy, J. of the IEST, 44(4): 24–27, 1998
11. S.J. Paley, “Cleanroom Wipers: State-of-the-Art Evaluation Techniques”, Cleanrooms, October 1996
12. H.R. Battacharjee and S.J. Paley, J. of the IES, 41(6), 1998, 19–25
13. H.R. Bhattacharjee et al, Scanning, 15 (5), 1993, 301–308
14. A. Shiue et al, Aerosol and Air Quality Research, 11, 2011, 460–465
15. EU Guidelines to Good Manufacturing Practice Medicinal Product for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal Products. The Rules Governing Medicinal Products in the European Union. Brussels: Commission of the European Communities, 2008, Vol. 4
16. C.F. Mattina, and S.J. Paley, J. of the IES, 34 (5), 1991, 21–28
17. A.P. Manian, et al, Textile Research Journal, 78 (8), 2008, 710–717
The author
A pharmaceutical microbiologist with more than 20 years of experience, Tim Sandle has written many papers and technical articles. He is a visiting tutor with the University of Manchester School of Pharmacy and is head of microbiology at BPL.




