The Coronavirus emergency has imposed companies new rules and instructions to prevent any risk of contagion and to ensure employees work in a safe and protected environment.
In order to do this AM Instruments reccomends programming interventions which aim to completely disinfect environments and surfaces, thus guaranteeing safe working spaces.
FDA Reccomendation
In “Good Manufacturing Practice Considerations for Responding to COVID-19 Infection in Employees in Drug and Biological Products Manufacturing”, June 2020, FDA offers a guidance representing the current thinking of the Food and Drug Administration (FDA or Agency) on this topic.
The document focus on manufacturing controls to prevent contamination of drug, and, specifically suggests, to help prevent transmission among employees and contamination of drugs/materials by a COVID-19-infected employee engaged in drug manufacturing at the workplace, drug manufacturers should:
- Clean and sanitise nonproduction areas (such as offices, elevators, break rooms, changing rooms, and restrooms) more frequently
- Update existing procedures to institute more frequent cleaning, sanitization, and/or sterilization of surfaces in the production areas, particularly surfaces that are contacted frequently, such as door handles, equipment latches, bench/counter tops, and control panels. Special attention should be paid to sanitizing/sterilizing equipment and product-contact surfaces.
- Consider expanding existing procedures to include using gloves, face masks, and/or gowning where such measures were not previously required.
- Consider further restrictions on employee access to any manufacturing area, beyond that required by CGMP regulations and recommended by Agency guidance18 and normal practice, to limit the possibility of contamination.
If a potential or actual viral contamination event is identified, drug manufacturers should promptly clean, disinfect, sanitize, and if necessary, sterilize affected equipment, surfaces, production areas, and facilities, before resuming manufacturing.
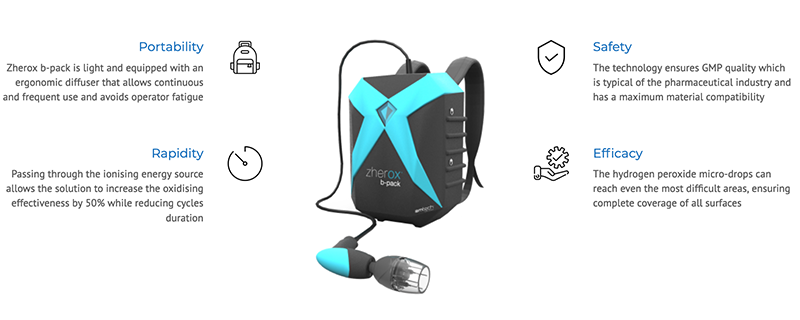
To learn more about Zherox b-pack, click here.




