Surfaces in the healthcare industry pose different levels of contamination risks based on the level of contact with the drug product. Typically, the types of surface are classified as direct, indirect, and non-product contact surfaces.
Surface cleanliness monitoring must be adapted based on the potential risk to contaminate the next product. Thus, the types of surfaces selected for monitoring should be scientifically justified; a walkthrough and observation of the surfaces to analyse potential exposure to the drug product are critical to support the justification.
The level of monitoring of an indirect product contact surface after a cleaning process may depend on the criticality of the surface
In this article, a direct product contact surface is one that would be in direct contact with the product (e.g. equipment, material, items) or any intermediate (e.g., media, buffer) that would enter in the final product composition. An indirect product contact surface is one that directly contacts a product contact surface and can transfer residues to the next product (e.g. stopper bowl, scissors). A non-product contact surface is one that would never be in contact with the product or indirect or direct product contact surfaces (e.g. floor, ceiling, the exterior of equipment).
Regulatory guidelines are comprehensive regarding cleaning monitoring requirements for direct product contact surfaces. For non-product contact surfaces, they are typically geared toward preventing contamination of the cleanrooms. For indirect product contact surfaces, however, the requirements regarding cleaning validation monitoring are not well developed.
Market research
This article is based on findings from a survey on several types of pharmaceutical manufacturers.
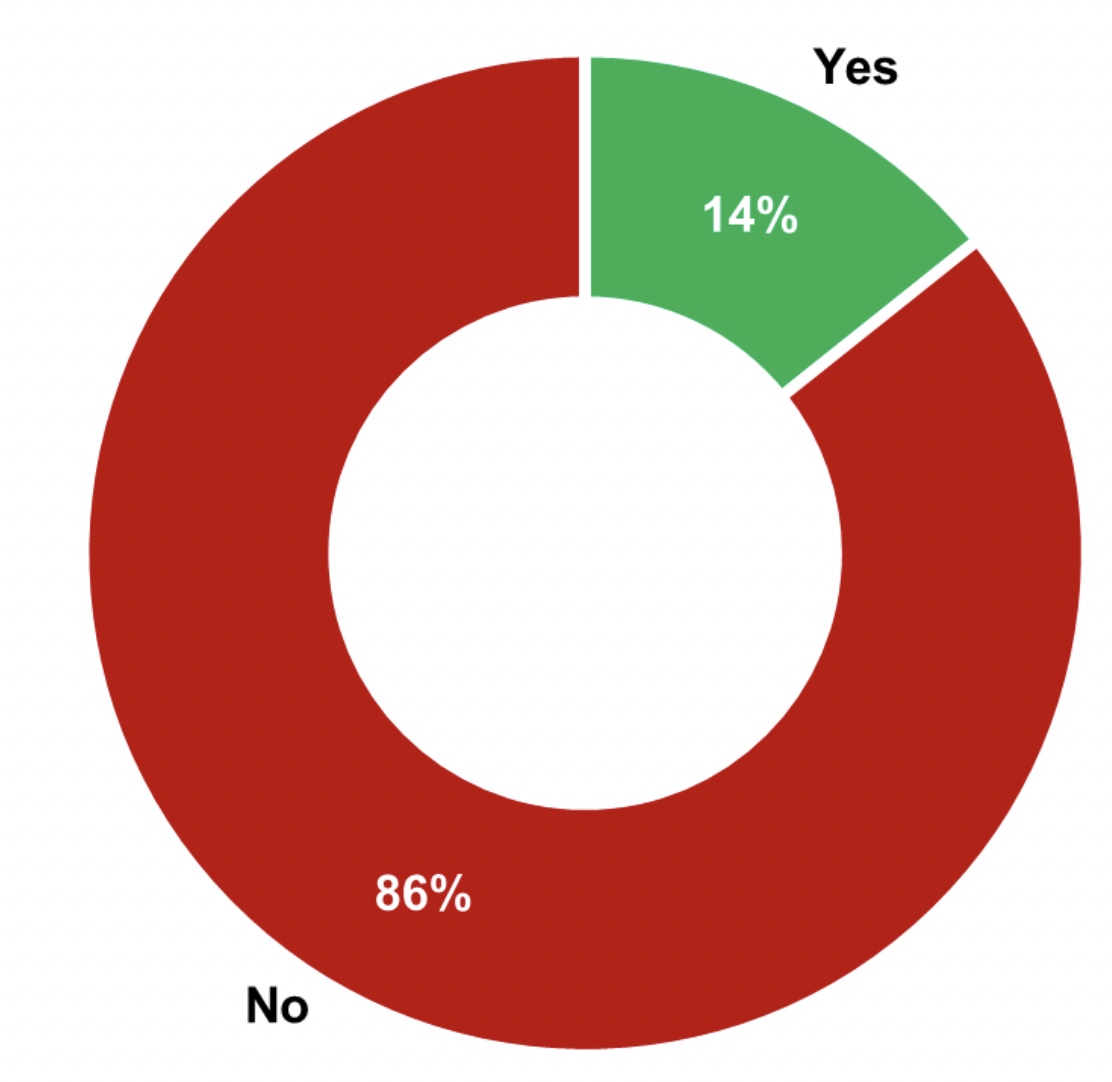
Figure 1: 86% of manufacturers have different monitoring requirements for direct and indirect product contact surfaces
The survey aimed to understand how indirect product contact surfaces are monitored: 35 European companies were canvassed; 57% of them non-sterile; 23% sterile; 11% vaccine; 3% medical device; and 6% hospital.
The survey discussed routine monitoring alone and not the control used during early clinical production, the initial validation, requalification, or revalidation. Based on the results, 86% of the manufacturers use different monitoring requirements determined by the surface type, while 14% use the same cleanliness requirements for both direct and indirect product contact surfaces (see Figure 1).
The surface type should be determined through a risk analysis where the product's risk of contamination is assessed. The risk-based assessment should be coupled with a walkthrough and visualisation of the production stream to confirm the type of surfaces.
Only 37% of the companies surveyed performed a risk-based analysis, coupled with walkthrough and visualisation of the production stream, to confirm the surface type. But, 63% determine the surface type based only on experience and visualisation of the different surfaces.
Which monitoring approach to choose?
It's common sense to have different monitoring requirements for a direct and indirect product contact surface. The level of monitoring should be defined based on the risk of altering the product or eventually affecting patient safety.
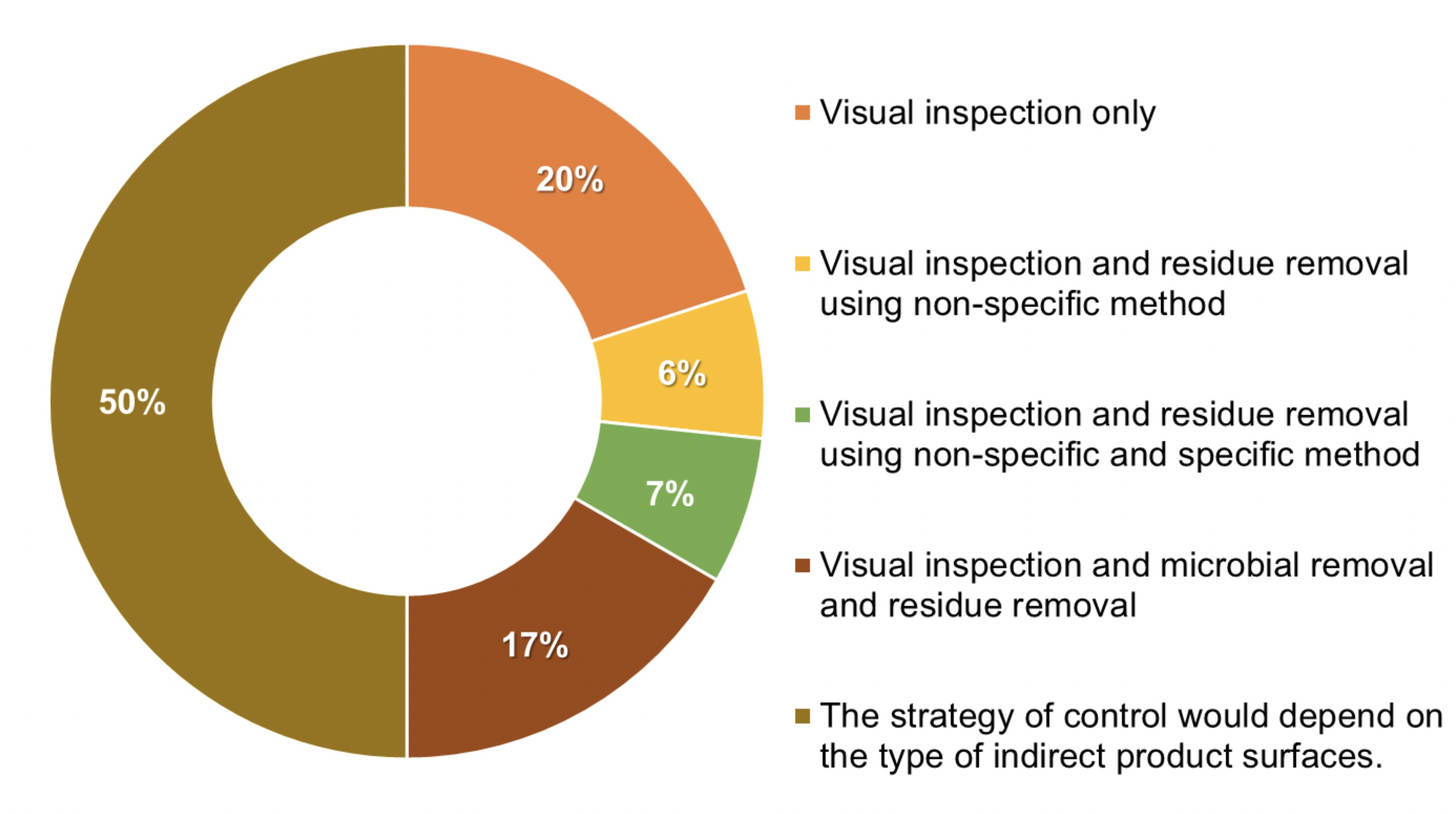
Figure 2: 50% of the interviewed manufacturers that answered “no” in Figure 1 are adapting the monitoring of indirect product contact surfaces based on their criticality.
Visual inspection for a non-product contact surface is enough. However, more stringent monitoring for direct product contact surfaces should be implemented. For indirect product contact surfaces, the monitoring strategy should be based on the potential risk to contaminate the product, or a direct product contact surface, which is a common practice in the industry.
From 30 of the manufacturers in Figure 1, 50% of them adapt their monitoring strategy based on the criticality of the indirect contact product surfaces (see Figure 2).
The monitoring approach for an indirect product contact surface may differ depending on the criticality of the surface and the position from the product or the process stream. Thus, it is possible to define different types of indirect product contact surfaces based on their criticality. Only 54% of the manufacturers surveyed define different types of indirect contact surface (see Figure 3).
The types of indirect product contact surface may be defined as follows:
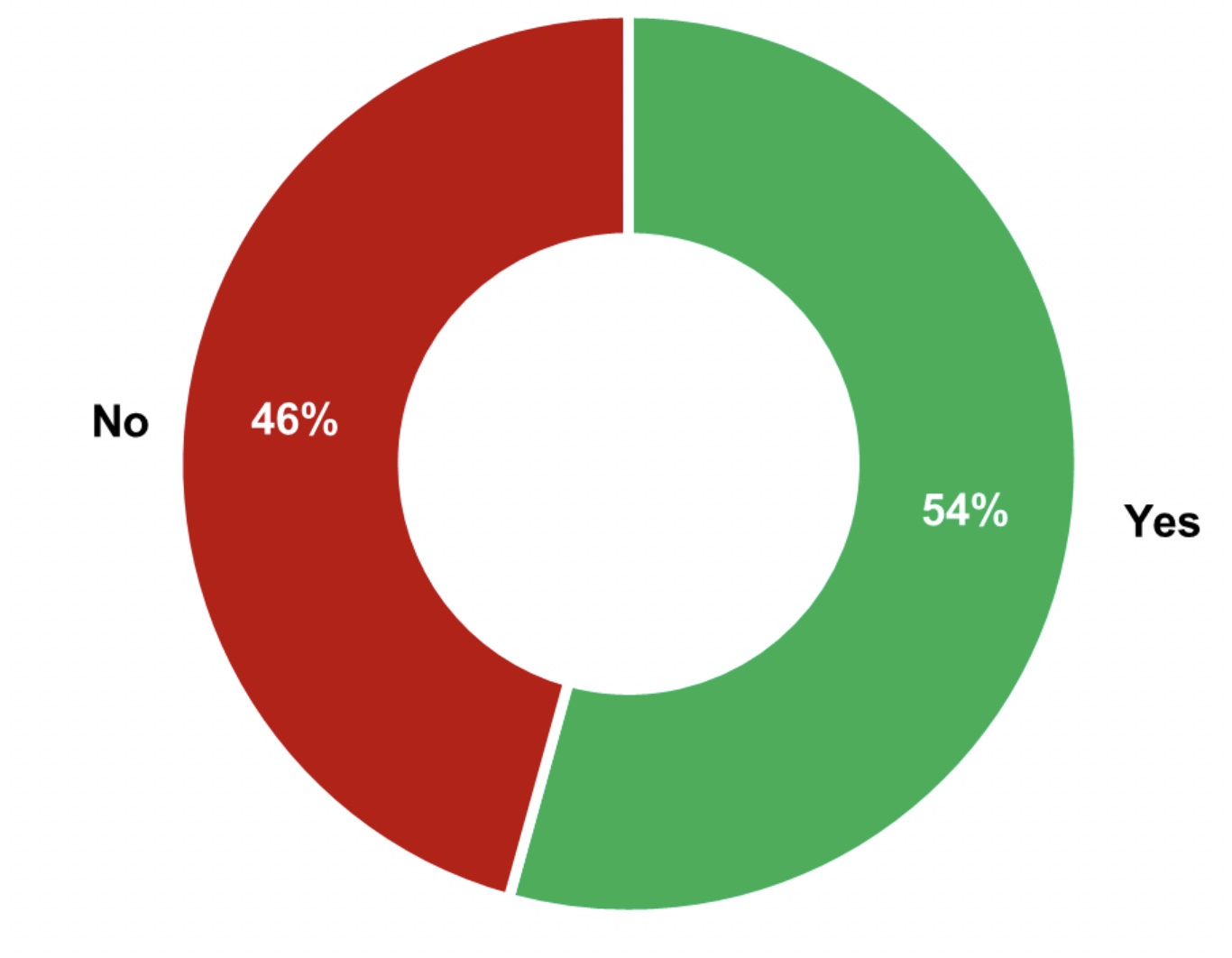
Figure 3: 54% of the interviewed manufacturers have defined different
types of indirect product contact surfaces
- High-risk indirect product contact surface is one that would be in contact with a direct product contact surface that is cleaned and/or sterilised. The indirect contact product surface may be in contact with the product residue during use (e.g. stopper bowl, spatula, and delivery chute in an isolator or RABS, and lyophiliser, if considered as an indirect contact product surface.
- Low-risk indirect product contact surface is one that would be in contact with a direct product contact surface that is not cleaned or not sterilised. The indirect product contact surface is not in contact with the product residue.
Thus, depending on the criticality of the indirect product contact surface, and the level of cleanness of the direct product contact surface, different monitoring approaches may be justified, as proposed in the decision tree (see Figure 4).
Special conditions
A walkthrough of the production stream and a visualisation of the surface is not always sufficient. The equipment usage and cleaning "life cycle" should be analysed to confirm the surface type and monitoring methods. For example, the external surface of a mobile vessel may be considered as a non-contact product surface. But, if this vessel is introduced into a washing machine, where both direct and indirect contact surfaces are also cleaned, specific monitoring should be set to confirm the absence of contamination, such as marker ink, adhesive present on the ticket) of a direct or indirect contact surface.
Real-case scenario
Let's consider case studies. Case #1 is based on a multiproduct large sterile manufacturer that disinfects its RABS after the filling of the batches in syringes is over. The disinfection procedure requests the operator to disinfect the RABS surface both the indirect and non-direct contact product. The operator would disinfect the surface using a wet wipe containing the sporicidal agent.
During a US FDA inspection, the regulator raised the following observation: "Cleaning validations have not been performed for the following equipment: the manual cleaning for the stopper bowl within filling line has not been validated." The manufacturer answered to the observation by implementing a manual cleaning of the stopper bowl using visual inspection. During validation, the bioburden limit and endotoxin testing were performed (see case 4 in Figure 4). Finally, after cleaning, disinfection using a sporicidal agent is performed and then rinsed.
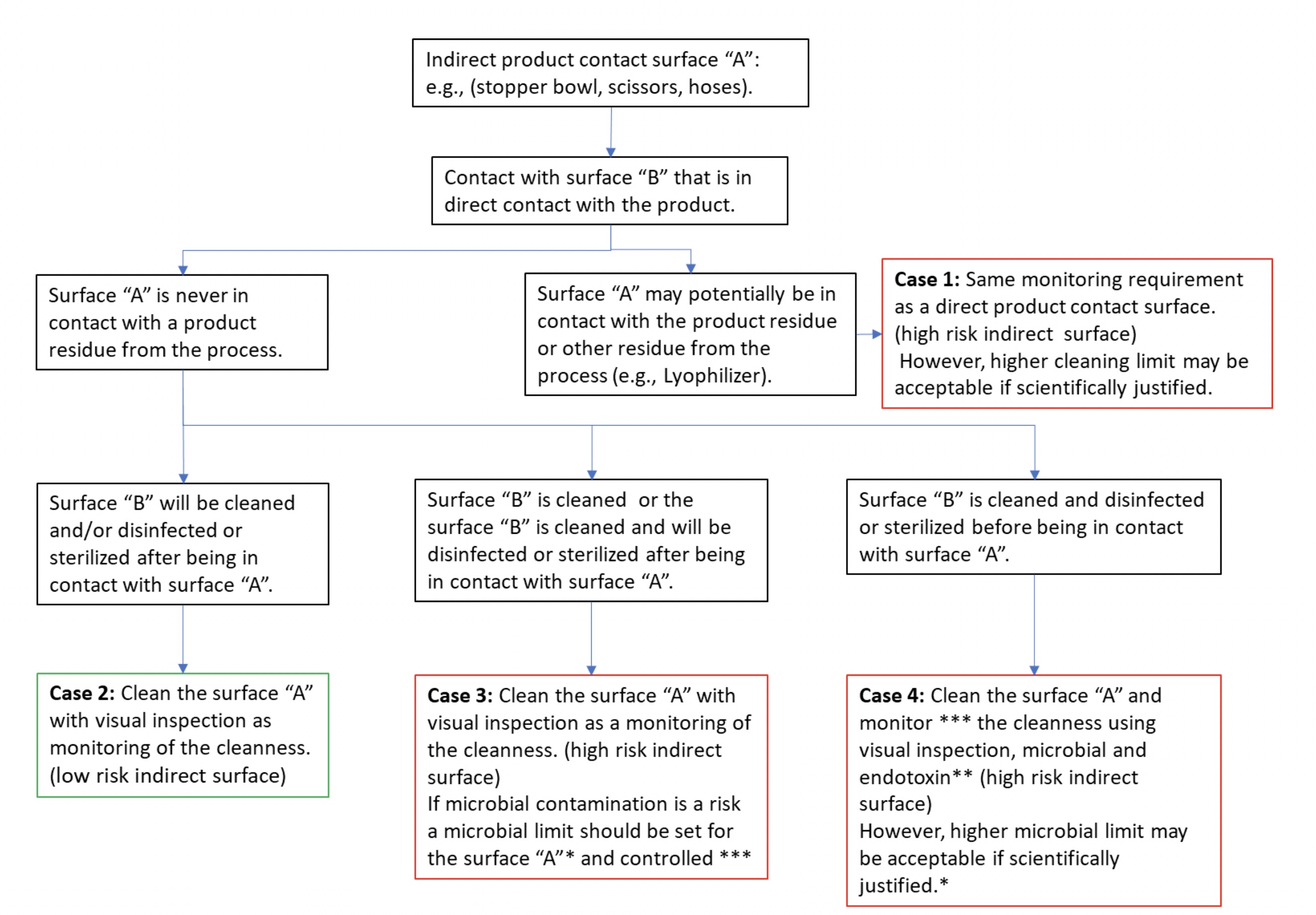
Figure 4: An example of a decision tree for indirect product contact surface monitoring
In case study #2, a multiproduct injectable drug manufacturing facility uses pliers and scissors to cut the hoses used to move bioburden control intermediate product from one vessel to another. The clean hoses are cut at a defined length based on the process need. Then, the hoses are sterilised before use. The material is then only disinfected using alcohol. After disinfection, the material sits in the cleanroom without being adequate protection from the environment.
A representative of the EMA raised this observation during an audit: "The small equipment, such as pliers and scissors, are only disinfected using alcohol without cleaning before use of the small equipment." The manufacturer addressed the observation by implementing a manual cleaning of the material, followed by wrapping to protect them from the environment before re-use (see case 3 of Figure 4). During the cleaning validation, microbial monitoring after materials cleaning was not performed as the risk of microbial contamination was not identified.
A walkthrough of the production stream and a visualisation of the surface is not always sufficient
Therefore, the level of monitoring of an indirect product contact surface after a cleaning process may depend on the criticality of the surface.
The criticality is based on the potential risk to contaminate a product or a direct contact surface. It would also vary depending on where the indirect product contact surface is positioned in the production stream. Thus, the criticality of an indirect product contact surface should be determined using scientific justification, coupled with a visual inspection of surfaces before batch and cleaning processing.
Different monitoring and limit approaches for indirect product contact surfaces are acceptable depending on their criticality. The frequency of monitoring should be scientifically justified, providing insight into why different approaches are being used at different manufacturer sites.
N.B. This article is featured in the November 2019 issue of Cleanroom Technology. Subscribe today and get your print copy!
The latest digital edition is available online.





