Cleanroom facilities for micro-electronics, pharma-biotech, medical devices and healthcare do differ significantly from high-care / high-risk food processing facilities. Still, food processing is an interesting area for professionals with a contamination background and vice versa.
In a world full of classifications and requirements (ISO-14644-1, EU-GMP, FDA, e.g.) the lack of distinct cleanliness requirements for the food industry is noticeable.
This is obviously because there are many different types and formats of food products.
As hygiene in food processing includes contamination control, the vast variety in substances, processes, and huge volumes make the food processing world take a different approach. The main differences will be addressed, the challenges discussed, and a follow-up case study article will illustrate the results.
Contamination control in the food industry?
Within the approach of the ISO TC209 on the ISO 14644, and ISO 14698 cleanroom standards, a ‘cleanroom’ has to have a classification by particle concentration in the air as per ISO 14644-1.
One might suppose that any room, not having such a classification, will not be of interest considering contamination control. Typically for food production plants, this is not the case.
Basic guidance on this is given in the Codex Alimentarius. This Codex’s standards are based on sound science provided by independent international risk assessment bodies or ad-hoc consultations organised by FAO and the WHO.
The code of practice “General Principles of Food Hygiene, CAC/RCP 1-1969 (2003) addresses contamination. It contains the following definitions:
- “Contaminant - any biological or chemical agent, foreign matter, or other substances not intentionally added to food which may compromise food safety or suitability.”
- “Contamination - the introduction or occurrence of a contaminant in food or food environment.”
In section IV on Establishment: Design and Facilities, these objectives are given:
“Depending on the nature of the operations, and the risks associated with them, premises, equipment and facilities should be located, designed and constructed to ensure that: contamination is minimized; design and layout permit appropriate maintenance, cleaning and disinfections and minimize air-borne contamination; surfaces and materials, in particular those in contact with food, are non-toxic in intended use and, where necessary, suitably durable, and easy to maintain and clean; where appropriate, suitable facilities are available for temperature, humidity and other controls; and there is effective protection against pest access and harbourage.
RATIONALE: Attention to good hygienic design and construction, appropriate location, and the provision of adequate facilities, is necessary to enable hazards to be effectively controlled.”
Typicals - Product-specific requirements
The essential aim of the Codex Alimentarius is to secure product safety for the customer. This is comparable to the pharma industry to the extent that the production has to be controlled, as well as the traceability from raw materials all the way to finished products.
Rather than clear levels of cleanliness that are given for pharmaceutical products (especially the aseptic ones), food products only require limited levels of certain pathogenic species are given.
This is very much in line with current thinking in EN 17141 on biocontamination control, where a difference is made between levels of specific harmful microbiology and the overall nonspecific non-harmful general microbiology level. This might add to the improvement of control and monitoring. An increase in product shelf life and the reduction of preservatives are drivers for better production processes and environmental control when exposed.
In recent years, additional substances have been identified as contaminants: allergens and in some cases endotoxins. This has already led to major changes in production facilities. It is essential to implement a worldwide quality management system that covers the essential quality and safety aspects. The approach used is usually HACCP (Hazard Analysis and Critical Control Points).
Typicals - Hygiene and cleaning and zoning
Focusing on process steps where contamination can occur the EHEDG (European Hygienic Engineering & Design Group) has defined principles for hygienic design. EHEDG Guideline 8 Hygienic design principles taking a look at process equipment and its surroundings.
Furthermore, levels of hygiene are identified. There is not a very stringent definition of those levels, as they are determined by the HACCP analysis. It has been identified that the design of the surroundings where product matter is or can be exposed needs to allow for the way the cleaning will be accomplished.
Three types of cleaning are identified: (a) Wet cleaning; such as high pressure, high temperature and/or high detergent water-based fluids, (b) Controlled wet cleaning; using wetted cloth or wipers and (c) Dry cleaning; using dry cloth or wipers.
A combination of each hygiene level and each cleaning procedure was used to identify zones in a facility. The typical zones are given in table 1.
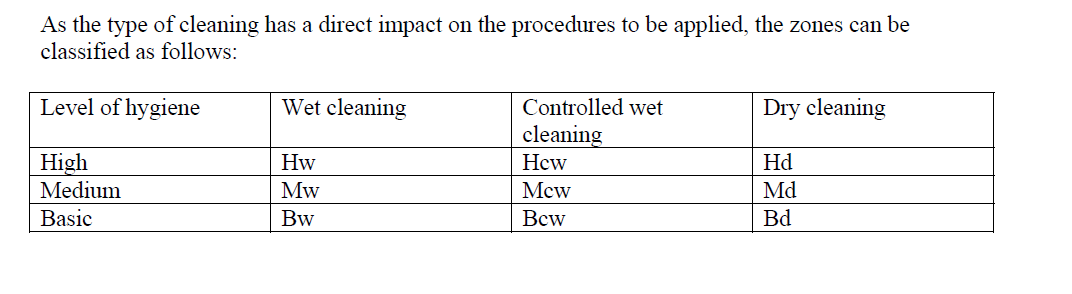
Table 1: Hygiene zones and cleaning methods from EHEDG Guideline 26 (withdrawn and integrated into Guideline 44 (under periodic review))
An important reason for this approach is the nutritious nature of all food products and the much larger quantities. Water and nutrition are the key factors to support the life of moulds, yeast, bacteria, and even rodents. For that reason, contamination control starts with landscaping and fencing around a facility to avoid easy intrusion of animals and insects, all the way through to gowning rooms, airlocks, towards areas of food processing.
Typicals - Reduction and (re-)contamination
To control microbial growth in raw materials and products, various reduction treatments are utilised, such as blanching, pasteurisation, sterilisation, additives, and pH changes. Apart from very specific cases such as parenteral food, all food products are not sterile. Sterility is not feasible because the taste and constitution of the product would be lost. As biocontamination continues to grow such reduction steps require stringent hygiene to avoid additional or recontamination. The effect is indicated in Figure 1.
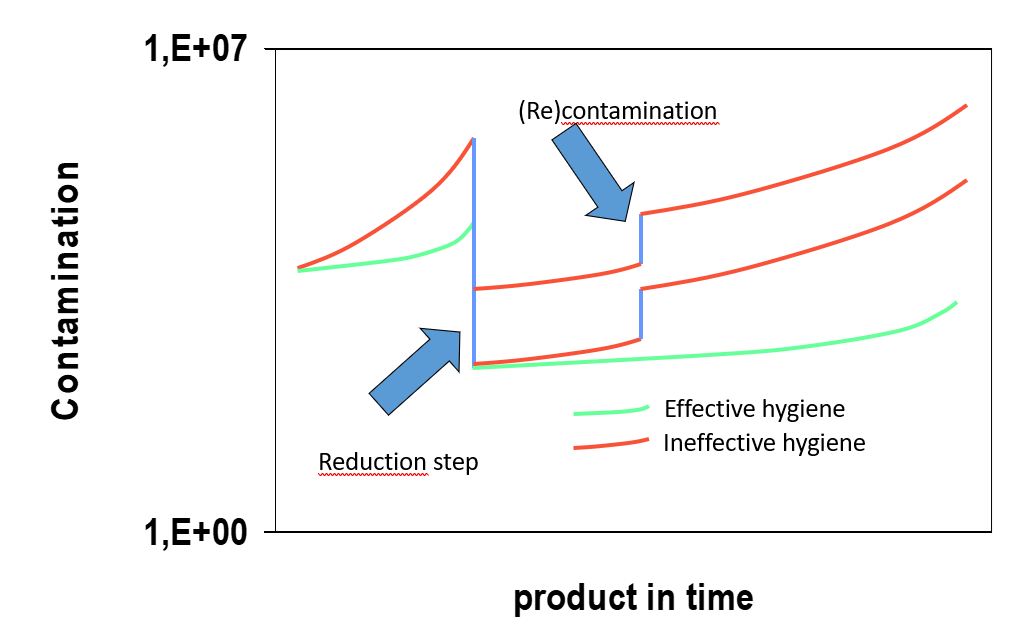
Figure 1: The effect of hygiene to control the level of biocontaminants
As control over the cleanliness of product when exposed to the environment is so important the best way is to reduce the area to be cleaned by minimising the exposure of the product.
Comparable to ISO 14644-4 Annex A figure A1 indicates zoning and material and personal flows a food facility can be designed.
Typicals - Cleanability
Product spilling can be abundant and wet cleaning is quite frequent in food processing facilities. Paying attention to cleanability, not only inside process equipment but outside as well, has made EHEDG define various guidelines on the hygienic construction of equipment and facilities (Figure 2).

Figure 2: Cleanable arrangements of control cabinet (Rittal) and cabling
This has much more focus than many pharma-biotech plants crowded with formulation vessels, bioreactors, and downstream processing equipment (Figure 3).

Figure 3: Un-cleanable bioreactor by congestion and poor design
The challenge then is to avoid any uncleanable areas and surfaces.
Where pharmaceutical plants, especially for sterile products, put emphasis on concealing as much as possible inside walls and above spaces in technical voids to reduce exposed areas that need to remain clean, a food production zone where some waste material is almost unavoidable, needs to have no hidden areas were pest nor microbial control can survive and grow. Therefore in hygienic design, piping, cabling, and equipment are all appropriately placed away from walls, ceilings etc. as to be able to clean them, or outside a critical hygiene zone at the other side of a boundary.
Typicals - The humidity challenge
Another important factor is the need to control the humidity and avoid condensation, as water is a very important factor in microbial growth. This implies that surfaces that are or can be cold below the dew point need to be well insulated.
At the same time, the relative humidity needs to be below a certain level. Especially when high-pressure high temperature water cleaning takes place. The impulse of the water will remove the soil but also adds vast amounts of life-giving water. Whenever these types of cleaning are needed only very solid walls and ceilings can be recommended.
Special care should be taken when recirculation is employed in the air handling and filters can become saturated. In such cases, HEPA filters can be found showing fungi growing through.
Typicals - Zoning
A more effective way is to separate equipment in such a way that it can be outside a zone of high classification. This requires a close study of the logistics of a production process. The various zones are segregated by physical barriers in combination with a pressure-flow cascade. A schematic example is given in Figure 4.
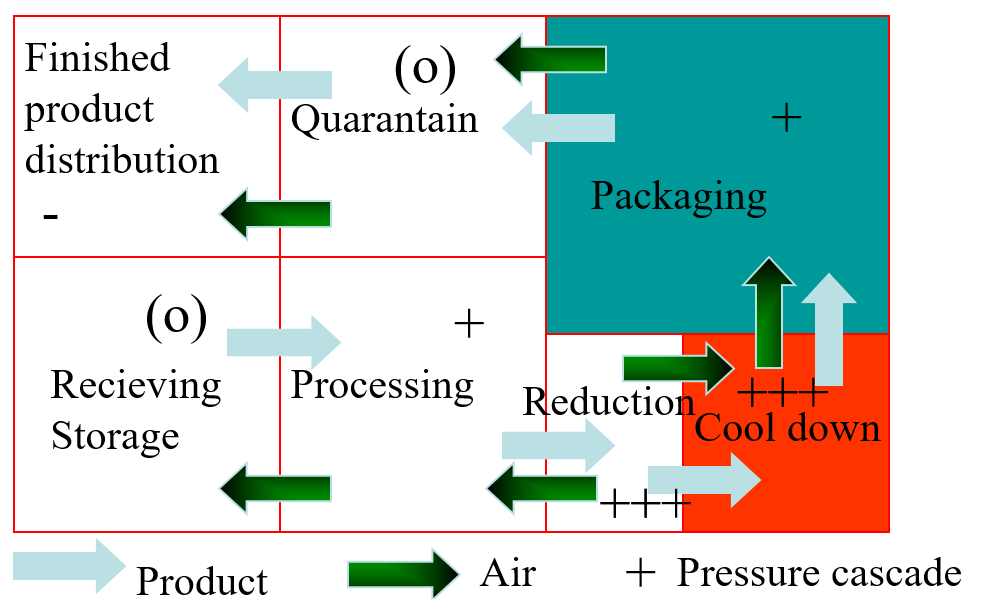
Figure 4: Schematic routing showing segregated zones and pressure flow cascade
